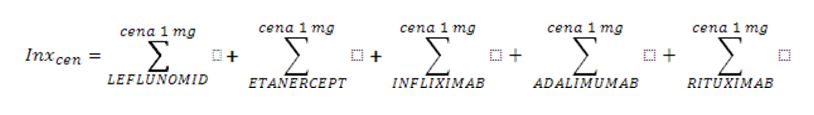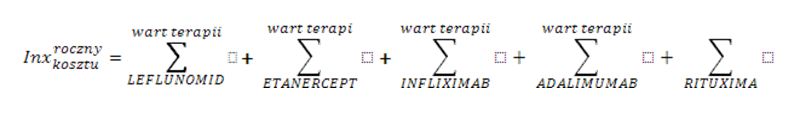The evaluation of the effectiveness of funding treatment programs in rheumatology
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Andrzej Śliwczyński |
1. Oddział Zdrowia Publicznego, Wydział Nauk o Zdrowiu, Uniwersytet Medyczny w Łodzi;2. Narodowy Fundusz Zdrowia; |
Background: The public payer in Poland has been financing biological drugs in rheumatology since 2004. Until now, there have been no analyses of the influence of this type of funding on the cost level for the public payer. Financing over an 8 year period allows an objective approach towards the results.
Materials and methods: Data extracted with the use of data tools reported to the National Health Fund (NFZ) by health service providers, including drugs used in a patient’s therapy. For data analysis, statistic tools were used: Statistica 9 and 10 and Excel spreadsheet.
Results: The number of people treated in Poland with biological drugs is approx. 5% of the potential population with rheumatoid joint inflammation diagnosis and juvenile idiopathic joint inflammation. In the analyses, the results of the Kobelt-Kasteng report have been confirmed, referring to the differences existing in Europe when it comes to therapy cost disparity. The cost of therapy in Poland increases depending on the type of therapy: infliximab (for patients up to 70kg), then rituximab and etanercept, adalimumab and infliximab (for patients over 70kg). The calculated price index and therapy cost indicates that the costs of such therapies are lower in Poland in comparison with other countries. The r-Pearson correlation factor of 0.61 to 0.73 indicates that there is an accurate balance between the number of clinics conducting the therapy and the number of patients. In the analyzed period, the budget for the rheumatology biological drug therapy increased.
Introduction
The development of medical technologies, both in the area of medical and drug technologies, allows better patient care and a more effective treatment of various diseases which have not had enough therapeutic options so far. At the same time, the public payer in Poland has been asked to cover additional expenses. In the organization of the health care system in Poland, which is financed entirely by the public payer, so-called therapeutic programmes have been introduced, which allow strict spending control, and they are considered to be a temporary stage before adding the drug to the reimbursement drug list. Financing new and expensive technologies in the form of a separate budget, meant for therapies of particular diseases, began in Poland in 2004. The first of such programmes was the rheumatoid joint inflammation program financed in the Silesian Province. In spite of the fact that the programme lasted for 8 years there are no publications which would allow an effective assessment of this form of payment for health care services, which makes one think, that an attempt to make such an assessment is a necessity. In rheumatology, financial settlement of health services in the form of therapeutic programmes was used in rheumatoid joint inflammation treatment with leflunomid and biological drugs (adalimumab, etanercept, infliksimab, rituksimab). The goal of this study was to analyze the health programme used in 2004 in various forms in rheumatology to assess:
1/ if the form of the therapeutic programme allows limiting therapy costs in the observed period of time;
2/ if the form of the therapeutic programme does not have any negative influence on the availability of the therapy and the drug distribution in particular regions of Poland;
3/ the level of the therapeutic programme budget use and regional differences.
Material and methods
Health services are financed by the National Health Fund in Poland (NFZ) on the basis of the Act and Health Ministry Ordinance [1,2,3]. Treating patients within the scope of rheumatological diseases on the therapeutic/drug programme is based on the health services contract on the conditions of hospital health service (so-called hospital contract). The organization, financing, and financial settlement of health services are specified for service contractors in the NFZ CEO fiats [4,5]. The financial settlement is based on an xml announcement specified by the Ministry of Health ordinance, which includes unique patient identification number (PESEL number) and the medical procedure code which was used with the particular patient [6,7]. The reporting has a hierarchical character, which means the code of the signed contract and the code of the medical procedure which was used are reported. The NFZ data has been analysed in terms of: contracts and reporting when it comes to contract realization for therapeutic/drug programmes in rheumatology. Analysis of the data related to the period 2004-2012, in which biological treatment in therapeutic programmes was funded. Different drugs are incorporated into the programme at different times and this is reflected in the description of tables and figures. In the search, SQL query has been used and computer application Business Object in 6.5 version and XI using a filter which is in accordance with the scope code (different for different years), for which contracts have been signed and the code of the medical procedure used (different in reference to various active substances). The data concerning the drug gross costs in particular countries was taken from IMS Health company (1st quarter of 2010). For the correlation analysis r-Pearson correlation factor has been used for the data set “Region population” vs. “Drug costs” and “Region population” vs. “The number of patients” included in the Excel spreadsheet and Statistica 9.0 program. In order to standardize the results concerning patient therapy costs in various European countries the index weights have been calculated according to the formula:
- for prices – the total price for 1mg of particular drugs (where cena=price)
- for the cost of annual therapy – the total price of the therapy with particular drugs (where roczny koszt=annual cost and wart terapii=the cost of the therapy)
The assumption which was made during the research is that a higher index value corresponds with higher patient therapy cost in the country regardless of the drug with which the therapy was conducted. In calculating the cost of the treatment, the treatment regimen defined in the Product Characteristics was used.
Results
The potential population of patients, with the assumption that the epidemiology will be similar to other countries, could account for approx. 60,000 people [8,9]. According to the information reported by the service providers, this is the number of patients who were diagnosed with M05, M06 or M08 (Table 1):
Table 1. Number of patients with diagnoses M05; M06; M08 between 2004-2012
| ICD-10 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
| M05 | 66 554 | 67 589 | 69 578 | 70 540 | 76 661 | 79 808 | 80 529 | 82 794 | 80374 |
| M06 | 27 174 | 26 991 | 28 056 | 28 442 | 31 552 | 33 564 | 34 024 | 35 620 | 33 871 |
| M08 | 3 031 | 3 258 | 3 266 | 3 199 | 3 284 | 3 422 | 3 467 | 3 638 | 3 411 |
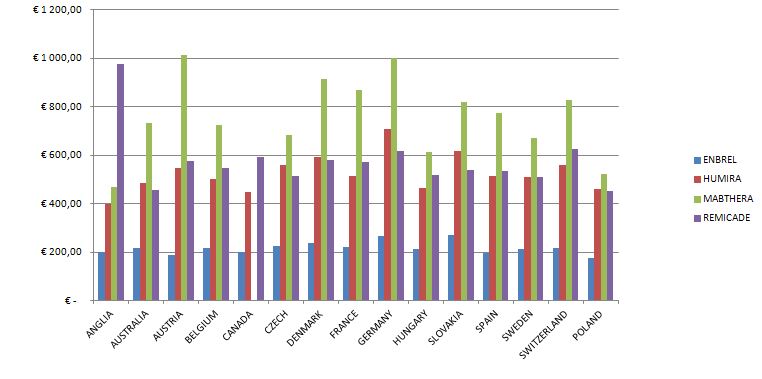
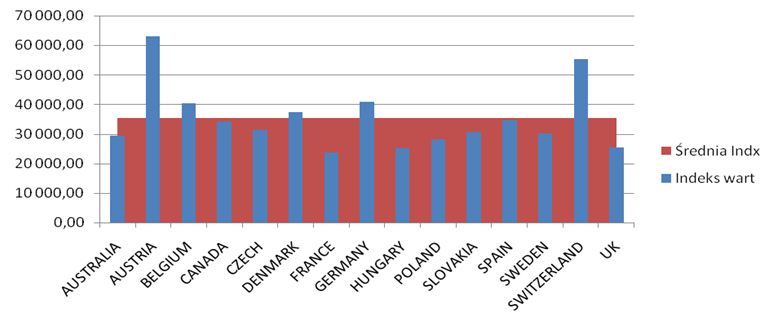
The number of all the patients in 2012 exceeded 80,000 people, but among this number there are people who were diagnosed with the disease and the therapy was not conducted (the diagnosis was then changed in the process). The conducted analyses of drug prices and costs revealed considerable differences, the biggest differences exist in Austria for biological drugs, the lowest in Great Britain (Etanercept), Hungary (Adalimumab, Rituximab) and France (Infliximab).The range of drug costs (the price of 1mg in Euro) between the countries chosen for the analysis is (with the minimal price basis): for Leflunomid approx. 650%; for Etanercept 224%; for Adalimumab 248%; for Rituximab 291%; for Infliximab 268%.
The data analysis has confirmed the results of the Kobelt-Kasteng report [10]. The difference in the average cost of treatment of a patient has been observed between Western Europe (12,900 Euro which is 52,995 PLN) and Central-Eastern Europe (3,750 € which is 15,405 PLN). The total cost of the Rheumatoid Joint Inflammation treatment (RZS) in Europe reaches € 25.1 billion (103.1 billion PLN).
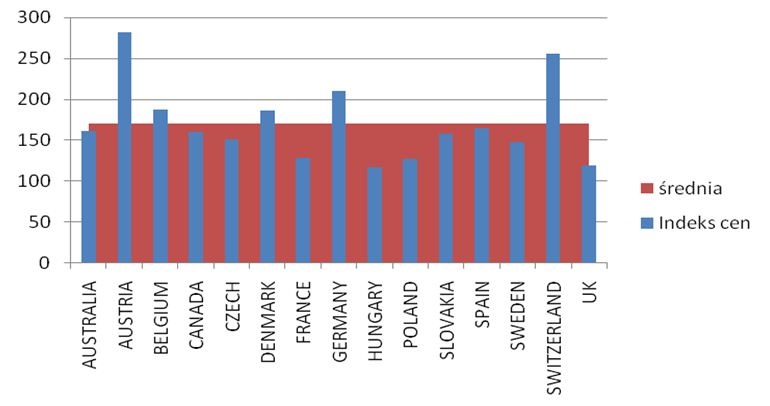
The calculated price index showed absolutely the highest value of drug prices in Austria, Switzerland and Germany ranked second, while the lowest price index occurs in England, Hungary and Poland. A comparison of therapy costs is possible with the use of the calculated price weight index. One can also use weights specified by the cost of one-shot and annual therapy. In the case of one-shot therapy per patient, the weight index of one-shot therapy has been calculated.
The highest cost of one-shot drug therapy is in Austria and Switzerland, the lowest one is in Great Britain and France. Costs of annual therapy with particular drugs depend on the price of the drug, the dosing method and, in the case of Infliximab, the patient’s weight.
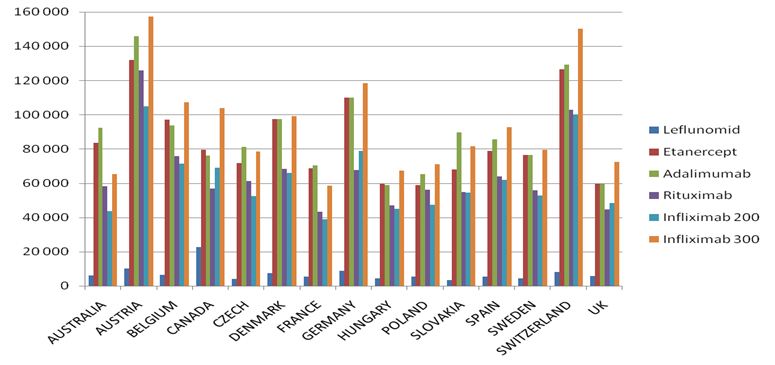
The highest costs of biological treatment per patient are in:
- Austria – adalimumab treatment is the most expenisve one among the analysed countries (over 145,000 PLN for annual therapy);
- Switzerland – adalimumab treatment is over 129,000 PLN for annual treatment;
- Germany – adalimumab treatment is over 12,000 PLN for annual treatment
The following, most costly medical procedures concern the use of etanercept in Austria (over 107,000 PLN), Switzerland (over 106,000 PLN) and Germany (over 96,000 PLN).
Therapies with individual active substances
The reports on the therapies using etanercept and infliximab in the years 2004-2005 was accounted for in the form of a monthly lump sum, so it is not possible to isolate the individual values. In the scope of the programmes, the population included in them continued to increase in 2010, reaching the number of about 6,000, which represents about 5% of the population in Poland.
Table 2. The number of patients, together with the kind of drug used, treated in rheumatology therapeutic programmes in 2004-2010
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
| ADALIMUMAB | 1 | 106 | 242 | 937 | |||
| ETANERCEPTUM | 621 | 1089 | 1350 | 1397 | 1730 | ||
| INFLIXIMAB, ETANERCEPT | 140 | 529 | |||||
| INFLIXIMABUM | 274 | 396 | 642 | 739 | 484 | ||
| LEFLUNOMID | 2328 | 2656 | 2686 | 2804 | 2764 | 2701 | |
| RITUXIMABUM | 13 | 178 | 337 | 401 | |||
| TOTAL | 140 | 2857 | 3551 | 4185 | 5080 | 5479 | 6253 |
An analysis, that takes into account the performance of services with the division into individual active substances, rendered the following results:
Leflunomide - the value of the funds amounted to 14% of the share in the rheumatology-dedicated programmes, the greatest funds - approx. 5.5 million PLN (2005-2005) were paid by the Masovian and Łódź divisions.
Table 3. The value of money spent on leflunomid therapy in particular provinces in 2004-2009
| NHF (National Health Fund) | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total |
| DOLNOŚLĄSKI | 191 140,00 | 409 021,20 | 346 720,40 | 509 924,78 | 592 737,60 | 693 705,12 | 2 743 249,10 |
| KUJAWSKO-POMORSKI | 180 880,00 | 313 740,00 | 406 800,00 | 413 580,00 | 475 680,60 | 505 549,20 | 2 296 229,80 |
| LUBELSKI | 183 350,00 | 329 023,80 | 464 612,05 | 560 077,80 | 477 511,80 | 430 117,48 | 2 444 692,93 |
| LUBUSKI | 54 340,00 | 73 962,00 | 37 494,60 | 67 370,68 | 47 493,16 | 44 826,08 | 325 486,52 |
| ŁÓDZKI | 612 560,00 | 1 001 019,60 | 999 329,76 | 1 051 606,92 | 980 087,32 | 849 844,12 | 5 494 447,72 |
| MAŁOPOLSKI | 422 940,00 | 976 778,40 | 916 781,84 | 867 567,04 | 843 840,20 | 832 936,26 | 4 860 843,74 |
| MAZOWIECKI | 884 260,00 | 981 348,00 | 819 550,20 | 834 393,00 | 936 924,00 | 1 115 581,56 | 5 572 056,76 |
| OPOLSKI | 168 720,00 | 242 906,40 | 305 683,06 | 362 208,60 | 348 036,00 | 352 521,00 | 1 780 075,06 |
| PODKARPACKI | 226 100,00 | 600 846,00 | 588 115,06 | 637 946,40 | 654 020,64 | 657 084,20 | 3 364 112,30 |
| PODLASKI | 168 340,00 | 182 498,40 | 132 264,00 | 109 848,00 | 112 320,00 | 96 876,00 | 802 146,40 |
| POMORSKI | 273 600,00 | 423 738,00 | 557 575,20 | 729 440,40 | 773 572,80 | 762 091,20 | 3 520 017,60 |
| ŚLĄSKI | 774 673,20 | 671 595,86 | 857 784,36 | 688 967,76 | 655 204,68 | 3 648 225,86 | |
| ŚWIĘTOKRZYSKI | 286 335,62 | 405 652,80 | 359 568,60 | 316 033,80 | 290 508,00 | 253 636,92 | 1 911 735,74 |
| WARMIŃSKO-MAZURSKI | 156 940,00 | 171 612,00 | 193 201,20 | 201 631,20 | 249 240,00 | 236 808,00 | 1 209 432,40 |
| WIELKOPOLSKI | 263 201,20 | 595 111,20 | 533 882,44 | 458 364,00 | 450 552,00 | 403 865,28 | 2 704 976,12 |
| ZACHODNIOPOMORSKI | 375 060,00 | 447 048,00 | 445 710,00 | 549 660,00 | 359 352,00 | 296 728,20 | 2 473 558,20 |
In total, approx. 2,600 patients were treated, the largest number of patients, i.e. approx. 2,000, were treated in the Masovian, Łódź and Lesser Poland divisions and the number of patients was the lowest in the Lubuskie division.
Table 4. The number of patients who were treated with leflunomid in a particular year and province in 2005-2010
| NHF (National Health Fund) | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
| DOLNOŚLĄSKI | 126 | 126 | 122 | 178 | 218 | 219 |
| KUJAWSKO-POMORSKI | 81 | 106 | 134 | 160 | 155 | 154 |
| LUBELSKI | 97 | 105 | 167 | 191 | 188 | 166 |
| LUBUSKI | 29 | 27 | 19 | 22 | 20 | 16 |
| ŁÓDZKI | 320 | 328 | 316 | 310 | 292 | 268 |
| MAŁOPOLSKI | 289 | 313 | 304 | 296 | 274 | 259 |
| MAZOWIECKI | 428 | 374 | 293 | 305 | 358 | 422 |
| OPOLSKI | 73 | 93 | 108 | 114 | 114 | 111 |
| PODKARPACKI | 147 | 174 | 190 | 205 | 208 | 215 |
| PODLASKI | 70 | 54 | 34 | 29 | 35 | 28 |
| POMORSKI | 186 | 133 | 213 | 223 | 247 | 259 |
| ŚLĄSKI | 324 | 287 | 279 | 258 | 215 | |
| ŚWIĘTOKRZYSKI | 112 | 115 | 107 | 92 | 88 | 80 |
| WARMIŃSKO-MAZURSKI | 52 | 47 | 58 | 63 | 68 | 73 |
| WIELKOPOLSKI | 136 | 159 | 166 | 155 | 123 | 121 |
| ZACHODNIOPOMORSKI | 182 | 180 | 170 | 183 | 119 | 97 |
| TOTAL | 2328 | 2658 | 2688 | 2805 | 2765 | 270 |
The highest incidence of the disease occurs at the age of 55 and women account for 83% of the patients. The average cost of the treatment amounted to 2,500-3,000 PLN.
Infliximab - the value of the funds spent on the treatment of patients was 47 million PLN (in the years 2006-2010). The highest amount of the funds (11 million PLN) was used in the Masovian Province and the Kuyavian-Pomeranian Province (9.3 million PLN) and it was the lowest in the Lubuskie Province (approx. 0.55 million PLN).
Table 5. The province participation in infliximab treatment budget spending in 2004-2010
| NHF (National Health Fund) | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total |
| DOLNOŚLĄSKI | 35 000 | 775 000 | 711 650 | 787 213 | 999 411 | 1 331 070 | 500 019 | 5 139 364 |
| KUJAWSKO-POMORSKI | 23 125 | 2 201 500 | 902 225 | 1 153 294 | 1 856 836 | 2 012 084 | 1 238 433 | 9 387 497 |
| LUBELSKI | 928 990 | 436 596 | 524 874 | 727 047 | 632 467 | 297 496 | 3 547 469 | |
| LUBUSKI | 120 000 | 215 000 | 32 500 | 18 478 | 8 455 | 111 926 | 51 978 | 558 337 |
| ŁÓDZKI | 5 000 | 592 970 | 117 125 | 132 189 | 163 460 | 738 900 | 593 766 | 2 343 411 |
| MAŁOPOLSKI | 530 000 | 1 370 000 | 435 000 | 587 133 | 859 894 | 928 846 | 952 721 | 5 663 594 |
| MAZOWIECKI | 1 056 235 | 3 168 500 | 1 455 755 | 1 295 916 | 1 236 186 | 1 638 335 | 1 422 778 | 11 273 705 |
| OPOLSKI | 315 000 | 315 000 | 240 000 | 265 265 | 240 973 | 190 242 | 118 373 | 1 684 853 |
| PODKARPACKI | 700 000 | 1 035 000 | 252 500 | 233 834 | 273 948 | 525 351 | 174 198 | 3 194 832 |
| PODLASKI | 335 000 | 566 820 | 220 000 | 344 375 | 600 376 | 741 063 | 464 275 | 3 271 909 |
| POMORSKI | 112 500 | 70 458 | 177 770 | 209 087 | 501 594 | 361 724 | 1 433 133 | |
| ŚLĄSKI | 265 000 | 277 440 | 684 987 | 1 511 475 | 1 045 800 | 3 784 703 | ||
| ŚWIĘTOKRZYSKI | 625 700 | 285 860 | 422 120 | 687 401 | 761 224 | 347 063 | 3 129 368 | |
| WARMIŃSKO-MAZURSKI | 510 150 | 136 848 | 184 807 | 355 296 | 247 127 | 141 646 | 1 575 873 | |
| WIELKOPOLSKI | 491 719 | 421 425 | 763 505 | 1 560 999 | 1 394 344 | 923 219 | 5 555 210 | |
| ZACHODNIOPOMORSKI | 467 900 | 447 348 | 759 393 | 583 697 | 392 427 | 2 650 764 |
The highest number of patients (556 persons) was treated in the Masovian Province (approx. 80 patients per year) and the Kuyavian-Pomeranian Province (419 patients, 26 persons per year). The lowest number of patients was treated in the Opole Province (75 persons in total).
Table 6. The number of patients treated with infliximab in 2004-2010
| NHF (National Health Fund) | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
| DOLNOŚLĄSKI | 3 | 41 | 37 | 40 | 64 | 73 | 30 |
| KUJAWSKO-POMORSKI | 10 | 68 | 46 | 55 | 83 | 92 | 65 |
| LUBELSKI | 36 | 18 | 28 | 42 | 40 | 21 | |
| LUBUSKI | 5 | 7 | 1 | 1 | 1 | 6 | 5 |
| ŁÓDZKI | 1 | 26 | 5 | 6 | 15 | 48 | 38 |
| MAŁOPOLSKI | 32 | 60 | 21 | 34 | 49 | 52 | 46 |
| MAZOWIECKI | 44 | 147 | 60 | 62 | 82 | 94 | 67 |
| OPOLSKI | 12 | 11 | 9 | 14 | 13 | 10 | 6 |
| PODKARPACKI | 23 | 32 | 9 | 12 | 17 | 26 | 13 |
| PODLASKI | 10 | 26 | 10 | 18 | 31 | 33 | 24 |
| POMORSKI | 9 | 3 | 14 | 14 | 23 | 18 | |
| ŚLĄSKI | 19 | 16 | 42 | 77 | 56 | ||
| ŚWIĘTOKRZYSKI | 19 | 10 | 17 | 38 | 36 | 19 | |
| WARMIŃSKO-MAZURSKI | 14 | 8 | 10 | 25 | 11 | 8 | |
| WIELKOPOLSKI | 22 | 18 | 42 | 83 | 80 | 49 | |
| ZACHODNIOPOMORSKI | 14 | 27 | 43 | 38 | 20 | ||
| TOTAL | 140 | 532 | 274 | 396 | 642 | 739 | 485 |
The distribution of the number of the patients according to their age indicates the dominance of patients aged 48-57. Women make up 79% of the patients. The average annual cost of the therapy amounted to 18,000-25,000 PLN per patient.
Etanercept - the value of the funds paid to service providers amounted to 166.7 million PLN in the years 2006-2010. The largest amount of the funds was used in the Silesian Province (22.2 million PLN) the Lesser Poland Province (18.9 million PLN) and the Masovian Province (1.1 million PLN) and the lowest was in the Lubuskie Province.
Table 7. The distribution of money spent on etanercept in 2006-2009
| NHF (National Health Fund) | 2006 | 2007 | 2008 | 2009 | 2010 | Total |
| DOLNOŚLĄSKI | 572 541 | 933 693 | 2 390 220 | 2 664 739 | 3 057 202 | 9 618 395 |
| KUJAWSKO-POMORSKI | 2 209 250 | 3 398 717 | 3 627 110 | 4 624 276 | 4 654 351 | 18 513 704 |
| LUBELSKI | 1 072 550 | 1 530 267 | 2 286 273 | 2 163 544 | 1 920 355 | 8 972 989 |
| LUBUSKI | 181 250 | 162 778 | 329 543 | 307 504 | 174 871 | 1 155 945 |
| ŁÓDZKI | 1 183 225 | 1 395 574 | 1 846 805 | 1 333 022 | 2 409 907 | 8 168 533 |
| MAŁOPOLSKI | 2 128 068 | 2 556 450 | 5 221 515 | 4 369 856 | 4 578 536 | 18 854 424 |
| MAZOWIECKI | 2 703 527 | 3 474 085 | 4 626 721 | 3 916 251 | 4 802 628 | 19 523 213 |
| OPOLSKI | 147 500 | 323 903 | 743 046 | 906 768 | 747 630 | 2 868 847 |
| PODKARPACKI | 1 105 155 | 1 434 205 | 1 854 487 | 1 385 340 | 1 492 133 | 7 271 320 |
| PODLASKI | 710 625 | 1 093 920 | 2 787 787 | 2 617 663 | 1 529 103 | 8 739 098 |
| POMORSKI | 323 589 | 761 116 | 1 359 052 | 1 426 795 | 1 380 922 | 5 251 475 |
| ŚLĄSKI | 2 134 100 | 3 152 328 | 6 396 265 | 4 728 711 | 5 778 752 | 22 190 157 |
| ŚWIĘTOKRZYSKI | 501 994 | 646 515 | 1 225 308 | 1 456 782 | 2 055 207 | 5 885 806 |
| WARMIŃSKO-MAZURSKI | 497 250 | 487 130 | 784 103 | 772 957 | 678 409 | 3 219 849 |
| WIELKOPOLSKI | 1 403 800 | 2 421 444 | 4 397 694 | 4 936 527 | 4 531 193 | 17 690 658 |
| ZACHODNIOPOMORSKI | 943 891 | 1 480 004 | 2 221 023 | 2 424 110 | 1 745 805 | 8 814 834 |
| TOTAL | 17 820 321 | 25 254 136 | 42 098 960 | 40 036 854 | 41 539 014 | 166 739 247 |
The highest number of patients was treated in the Silesian Province (approx. 200 patients per year, 864 in total) and the Masovian Province (170 patients per year, 815 patients in total).
Table 8. The number of patients treated with etanercept in 2006-2010 in particular provinces
| NHF (National Health Fund) | 2006 | 2007 | 2008 | 2009 | 2010 |
| DOLNOŚLĄSKI | 26 | 57 | 96 | 109 | 136 |
| KUJAWSKO-POMORSKI | 76 | 103 | 123 | 128 | 187 |
| LUBELSKI | 38 | 61 | 75 | 81 | 93 |
| LUBUSKI | 6 | 9 | 12 | 10 | 7 |
| ŁÓDZKI | 32 | 52 | 48 | 62 | 101 |
| MAŁOPOLSKI | 72 | 134 | 161 | 162 | 182 |
| MAZOWIECKI | 94 | 168 | 179 | 168 | 206 |
| OPOLSKI | 5 | 16 | 25 | 23 | 25 |
| PODKARPACKI | 30 | 41 | 58 | 61 | 70 |
| PODLASKI | 29 | 57 | 77 | 83 | 77 |
| POMORSKI | 13 | 38 | 44 | 48 | 62 |
| ŚLĄSKI | 101 | 165 | 187 | 176 | 235 |
| ŚWIĘTOKRZYSKI | 12 | 20 | 36 | 51 | 65 |
| WARMIŃSKO-MAZURSKI | 16 | 17 | 23 | 24 | 29 |
| WIELKOPOLSKI | 40 | 101 | 137 | 139 | 179 |
| ZACHODNIOPOMORSKI | 34 | 52 | 70 | 74 | 77 |
Etanercept is the only biological drug approved for the treatment of children, so there are two predominating groups: children at approx. 15 years of age and adults at the age of 56 years. Female patients dominate both the population of children (70%) and adults (69%). The average annual cost of the therapy amounted to 24,000-32,000 PLN per patient.
Adalimumab - was funded by the therapeutic programme from the end of 2007. At that time, 23.53 million PLN was spent in total for the treatment with this molecule, the largest amount of the funds was used in the Silesian Province (3.6 million PLN, 15.33%), the Masovian Province (3 million PLN, 12.9%) and in the Lesser Poland Province (3 million PLN, 11.65%) and the lowest amount (0.14 million PLN) was spent in the Lubuskie Province.
Table 9. The value of the money reported as a cost of the adalimumab therapy in particular provinces
| NHF (National Health Fund) | 2007 | 2008 | 2009 | 2010 | Total |
| DOLNOŚLĄSKI | 175 402,50 | 443 152,50 | 1 117 200,00 | 1 735 755,00 | |
| KUJAWSKO-POMORSKI | 241 552,50 | 321 300,00 | 1 600 200,00 | 2 163 052,50 | |
| LUBELSKI | 2 100,00 | 153 300,00 | 396 900,00 | 705 600,00 | 1 257 900,00 |
| LUBUSKI | 35 700,00 | 31 500,00 | 69 300,00 | 136 500,00 | |
| ŁÓDZKI | 149 100,00 | 914 130,00 | 1 063 230,00 | ||
| MAŁOPOLSKI | 174 300,00 | 678 300,00 | 1 892 100,00 | 2 744 700,00 | |
| MAZOWIECKI | 281 400,00 | 726 705,00 | 2 030 805,00 | 3 038 910,00 | |
| OPOLSKI | 16 800,00 | 117 600,00 | 273 000,00 | 407 400,00 | |
| PODKARPACKI | 12 600,00 | 159 600,00 | 745 500,00 | 917 700,00 | |
| PODLASKI | 119 700,00 | 214 200,00 | 497 700,00 | 831 600,00 | |
| POMORSKI | 155 400,00 | 541 800,00 | 848 400,00 | 1 545 600,00 | |
| ŚLĄSKI | 199 500,00 | 544 425,00 | 2 868 652,50 | 3 612 577,50 | |
| ŚWIĘTOKRZYSKI | 8 400,00 | 168 000,00 | 535 500,00 | 711 900,00 | |
| WARMIŃSKO-MAZURSKI | 168 000,00 | 168 000,00 | |||
| WIELKOPOLSKI | 191 100,00 | 827 400,00 | 1 507 800,00 | 2 526 300,00 | |
| ZACHODNIOPOMORSKI | 21 000,00 | 256 200,00 | 422 205,00 | 699 405,00 |
The average annual cost of the therapy amounted to approx. 16,000-23,000 PLN per patient.
Table 10. The number of patients treated with adalimumab in 2007-2010
| NHF (National Health Fund) | 2007 | 2008 | 2009 | 2010 |
| DOLNOŚLĄSKI | 11 | 30 | 77 | |
| KUJAWSKO-POMORSKI | 11 | 11 | 104 | |
| LUBELSKI | 1 | 5 | 20 | 38 |
| LUBUSKI | 1 | 1 | 3 | |
| ŁÓDZKI | 7 | 54 | ||
| MAŁOPOLSKI | 10 | 24 | 93 | |
| MAZOWIECKI | 20 | 31 | 111 | |
| OPOLSKI | 2 | 5 | 10 | |
| PODKARPACKI | 2 | 8 | 41 | |
| PODLASKI | 5 | 7 | 30 | |
| POMORSKI | 8 | 17 | 34 | |
| ŚLĄSKI | 10 | 39 | 178 | |
| ŚWIĘTOKRZYSKI | 1 | 11 | 31 | |
| WARMIŃSKO-MAZURSKI | 12 | |||
| WIELKOPOLSKI | 16 | 24 | 94 | |
| ZACHODNIOPOMORSKI | 4 | 7 | 27 | |
| TOTAL | 1 | 106 | 242 | 937 |
The largest number of patients was treated in the Silesian Province (227 persons), the Masovian Province (162 person) and the number of patients was the lowest in the Lubuskie Province (5). Women predominate among the patients and the predominating age of the patients was 55.
Rituximab - this molecule was the second-line treatment after using previous therapeutic options. Since 2007, 24 million PLN was spent on it, the highest amount of the funds, i.e. 4.3 million PLN was used in the Masovian Province and not much less was used in the Lesser Poland Province (2.9 million PLN) and the lowest share of the funds (0.28 million PLN) was used in the Lubuskie Province.
Table 11. The value of money spent on the rituximab therapy on the therapeutic programmes with the division into provinces
| NHF (National Health Fund) | 2007 | 2008 | 2009 | 2010 | Total |
| DOLNOŚLĄSKI | 24 450,00 | 255 092,24 | 500 955,00 | 897 315,00 | 1 677 812,24 |
| KUJAWSKO-POMORSKI | 481 880,00 | 825 750,00 | 760 791,00 | 2 068 421,00 | |
| LUBELSKI | 124 755,00 | 550 500,00 | 584 631,00 | 1 259 886,00 | |
| LUBUSKI | 22 020,00 | 66 060,00 | 187 170,00 | 275 250,00 | |
| ŁÓDZKI | 256 725,00 | 220 200,00 | 451 410,00 | 928 335,00 | |
| MAŁOPOLSKI | 85 575,00 | 584 445,00 | 902 930,10 | 1 381 755,00 | 2 954 705,10 |
| MAZOWIECKI | 122 300,00 | 894 073,00 | 1 398 270,00 | 1 915 740,00 | 4 330 383,00 |
| OPOLSKI | 95 370,00 | 154 140,00 | 264 240,00 | 513 750,00 | |
| PODKARPACKI | 12 225,00 | 156 495,00 | 363 330,00 | 550 500,00 | 1 082 550,00 |
| PODLASKI | 238 500,00 | 121 110,00 | 214 695,00 | 574 305,00 | |
| POMORSKI | 24 450,00 | 73 350,00 | 110 100,00 | 99 090,00 | 306 990,00 |
| ŚLĄSKI | 24 450,00 | 649 290,00 | 781 710,00 | 1 376 250,00 | 2 831 700,00 |
| ŚWIĘTOKRZYSKI | 239 720,00 | 330 300,00 | 456 915,00 | 1 026 935,00 | |
| WARMIŃSKO-MAZURSKI | 81 950,00 | 154 140,00 | 220 200,00 | 456 290,00 | |
| WIELKOPOLSKI | 495 360,00 | 836 760,00 | 1 255 140,00 | 2 587 260,00 | |
| ZACHODNIOPOMORSKI | 92 960,00 | 484 440,00 | 330 300,00 | 907 700,00 |
The highest number of patients was treated in the Masovian Province (approx. 50 patients per year), and the Lesser Poland Province ranked second (approx. 30 patients per year, followed by the Silesian Province (111 in total) and the Greater Poland Province (approx. 100 patients).
Table 12. The number of patients treated with rituximab on rheumatology therapeutic programmes in 2007-2010
| NHF (National Health Fund) | 2007 | 2008 | 2009 | 2010 |
| DOLNOŚLĄSKI | 1 | 10 | 28 | 39 |
| KUJAWSKO-POMORSKI | 13 | 34 | 26 | |
| LUBELSKI | 6 | 23 | 25 | |
| LUBUSKI | 1 | 3 | 6 | |
| ŁÓDZKI | 11 | 10 | 19 | |
| MAŁOPOLSKI | 4 | 23 | 43 | 48 |
| MAZOWIECKI | 5 | 31 | 52 | 62 |
| OPOLSKI | 4 | 7 | 11 | |
| PODKARPACKI | 1 | 6 | 17 | 20 |
| PODLASKI | 11 | 6 | 8 | |
| POMORSKI | 1 | 4 | 5 | 6 |
| ŚLĄSKI | 1 | 26 | 37 | 47 |
| ŚWIĘTOKRZYSKI | 7 | 12 | 17 | |
| WARMIŃSKO-MAZURSKI | 3 | 5 | 8 | |
| WIELKOPOLSKI | 18 | 36 | 46 | |
| ZACHODNIOPOMORSKI | 4 | 19 | 13 |
The average annual number of patients treated with rituximab was approx. 230. There are two peaks in the dominant numbers of patients: the first one at the age of approx. 26 and the other at the age of 58; there are more women among the patients - 83.5%. The average cost of the treatment for one patient is slightly higher than 25,000 PLN.
Discussion
The prices of the drugs under analysis in Poland fall within the range of the lowest prices in Europe (3-4 rank in this respect), apart from rituximab, which is available at a price close to the average price in Europe. The cheapest is the annual infliximab therapy in patients weighing less than 70 kg. Lower prices of biological drugs in Poland are the results of the adoption of the negotiation system by the National Health Fund and the Ministry of Health that made it possible, during the analyzed period, to cut the prices of drugs, which led to a reduction in the annual cost of treatment per patient. Infliximab is followed by rituximab and etanercept and then adalimumab and the therapy with infliximab in patients weighing over 70 kg is the most expensive. The number of centres conducting the treatment within the therapeutic programme gradually increased in the period under analysis from 34 to 81. The number of centres was correlated with the population of the individual provinces at the correlation coefficient ranging from 0.61 to 0.73. The value of renegotiated therapeutic contracts (which were subsequently performed) increased from 4,964,882 PLN in 2004 to 89,804,313 PLN in 2009, the value of the used budget was lower and amounted from 3.1 mln PLN in 2004 to 75.5 mln PLN in 2009. It amounted to, relatively, 62.5% in 2004 and 84% in 2009 of the budget. The highest expenses per inhabitant were incurred in the Kuyavian-Pomeranian Province and in the Podlasie Province, the expenses were the lowest in the Lubuskie Province and the Pearson correlation coefficient ranged from 0.46 to 0.88 for the individual provinces and substances, indicating similar expenses and physicians’ preferences forced by the programme. The expenditure for therapeutic programmes ranged from 1.18% to 18.53% of the total rheumatology expenditure in various provinces and years. The number of patients treated within the programme increased from 140 in the year 2004 to 5864 in the year 2010. Women accounted for approx. 75-80% of the patients, usually at an age ranging from 50 to 60 (37%). Leflunomide was the most frequently used drug (the largest number of patients in 2009 was 2804), etanercept was the most frequently used biological drug (the largest number of patients in 2010 was 1730), followed by adalimumab (937 patients in 2010), infliximab was used less frequently (739 patients in 2009) and rituximab (401 patients in 2010). Etanercept had the highest reimbursement value (166,739,247 PLN), and the reimbursement value was the lowest for adalimumab (23,560,530 PLN). The unit cost of the therapy incurred by the payer per patient treated since 2008 has remained practically stable and it amounts to 13,500 PLN, despite inflation observed in this period (less than 3,000 PLN for leflunomide, 20,000 PLN for infliximab, 27,000 PLN etanercept, 18,000 PLN for adalimumab and 25,000 PLN for rituximab).
Summary
As a result of the analysis, it was shown that:
1/ The therapeutic programme kept a stable cost of the therapy over the observed period of time. The organization of the health provision having a clearly defined framework allows the use of high-cost treatments for patients more efficiently and precise definition of the population allows all stakeholders to achieve their goals.
2/ The form of the therapeutic programme did not have any negative influence on the distribution and use of the drugs in individual regions of Poland. In the analyzed period, the number of patient treatment in therapeutic programmes grew steadily.
3/ It has been shown that 90% of the budget dedicated to the programme was used despite differences between regions. In order to achieve efficiency in the use of resources, the contract value must be correlated with the ability of health providers and the population size in the region. Healthcare providers (hospitals) are utilizing the granted resources in the optimal way.
- Act of August 27, 2004 on healthcare services financed from public funds (Journal of Laws of 2008 No. 164, item. 1027, as amended)
- Regulation of the Minister of Health of January 11, 2010, amending the regulation on guaranteed benefits in health care programs (Journal of Laws of 2010 No. 05, item. 29, as amended)
- Regulation of the Minister of Health of March 2, 2010, amending the regulation on guaranteed benefits in hospital treatment (Journal of Laws of 2010 No. 30, item. 157, as amended)
- Order No. 101/2007/DGL 05.11.2007, amending the order on approval of "Specific information materials on the subject of proceedings to finalise contracts for providing health care services and on performance and funding of such contracts in specific fields, such as hospital treatment"
- Order No. 65/2009/DGL of President of the National Health Fund of June 19, 2008 on conclusion conditions and performance of contracts, such as hospital treatment contracts in the scope of therapeutic health programs
- Order No. 103/2012/DSOZ of President of the National Health Fund of 24 December 2012 on detailed XML reporting from outpatient and inpatient service performance
- http://www.msw.gov.pl/portal/pl/381/32/PESEL.html
- Nandi P., Kingskey G., Scott D.: Disease-modifying antirheumatic drugs other then methotrexate in rheumatoid arthritis and seronegative arthritis. Curr Opin Rheumatol, 2008; 20: 251-6
- Sokka T., Kautainen H., TolozaS i wsp.: QUEST-RA,: quantitative clinical assessemnet of patients with rheumatoid arthritis seen in standard rheumatolology care in 15 countries. Ann. Rheum. Dis. 2007; 66: 1491-96
- Kobelt G., Kasteng F. A raport prepared for the European Federation of Pharmaceutical industry associations (EFPIA) 2009; 2: 2