Pharmacoeconomic evaluation of fixed-dose triple combination for antihypertensive therapy in Ukraine
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
In Ukraine, the efficacy of treatment of arterial hypertension is only 19% in urban areas and 8 % in rural populations. The most important reasons of low efficiency of antihypertensive therapy (AHT) are a wrong choice of tactics of the patient management and low adherence of patients to treatment. The latter decreases with increasing amounts of prescribed drugs. One possible way to increase patients’ compliance to treatment and the effectiveness of therapy is to use fixed-dose combinations (FDCs) of antihypertensive drugs (AHDs). The share of FDCs consumption (in terms of DDDs/1000/day) in Ukraine in the total structure of AHDs consumption is 25%, which is significantly less than the proportion of patients (60%), requiring combined AHDs. This is an indirect evidence of low compliance of Ukrainian patients to HD treatment and the need of pharmacoeconomic study of benefits of antihypertensive therapy using FDCs. As a result of pharmacoeconomic cost-effectiveness analysis it has been found that antihypertensive therapy in patients with moderate and severe AH using triple FDC Val+Aml+HCTZ compared with three dual FDC: Val+HCTZ, Val+Aml, Aml+HCTZ provides greater clinical efficacy (the number of patients with the achieved target level of blood pressure). This triple FDC Val+Aml+HCTZ has pharmacoeconomic benefits (greater cost efficiency), compared with only one dual FDC Val+HCTZ. This gives the opportunity to save money, presents additional advantages in efficiency and justifies benefits from its use by hypertensive patients in need of appointing the third AHD CCB amlodipine in addition to the existing dual one using valsartan and hydrochlorothiazide.
Introduction
Arterial hypertension (AH) is the leading cause of death from cardiac diseases which defines a high social significance of the problem of treatment of this disease [1,2].
In spite of the wide range of antihypertensive drugs (AHDs) in the pharmaceutical market of Ukraine, only a small proportion of patients with hypertension are treated effectively. Effectiveness of the treatment is only 19% in urban areas and 8 % in rural populations [2], in Russia the frequency of achieving the target level of blood pressure (BP) is 21.5% [1]. The most important reasons of low efficiency of antihypertensive therapy (AHT) are a wrong choice of tactics of the patient management and low adherence of patients to treatment. To find adequate therapy in patients at high risk of cardiovascular complications is the most difficult natter. Results of multicenter clinical studies confirm that the achievement of target BP values of less than 130 and 80 mm Hg are observed in 10-12% of patients with diabetes mellitus and no more than 17 % of patients with renal failure [3,4]. Such a low level of the target BP imposes special requirements on the selection of AHDs. Antihypertensive monotherapy is effective not more than in a half of patients with a moderate increase in BP. ALLHAT studies have proved that only 60% of patients with AH of 1-2 degree reach the target BP values with monotherapy [4]. Frequency of use of a combination therapy in patients with hypertension of 2-3 degree is from 45 % to 93% [5, 6]. The most extensive trial HOT showed that to achieve DBP level less than 90 mm Hg the combination therapy was required in 63% of cases, and to achieve DBP less than 80 mm Hg – in 74 % of cases [7].
More pronounced effect of the combined AHT is due to different mechanisms of action of drugs to be combined, which solves the problem of AH multiple factors. The simultaneous use of different classes of drugs can influence the several links of AH pathogenesis – the activation of the renin-angiotensin-aldosterone and sympathoadrenal systems, endothelial dysfunction and renal impairment, myocardial hypertrophy and hypertrophy of the vascular wall [1,2,8]. The combination therapy allows ensuring the BP effective control on a background of good endurance without increasing doses of preparations. STRATHE study showed that the use of the combination therapy allows achieving the desired effect from the very beginning of the treatment of hypertensive patients [9].
One drawback of the AH combination therapy is regime complication and increased cost of treatment, since the patient should administer at least two medicines, the multiplicity prescriptions of which may be different. The use of fixed-dose combinations (FDCs) of AHD allows leveling the problem. Fixed-dose combinations reduce the number of tablets taken and enhance patients’ adherence to treatment, which is an important factor of its effectiveness. The advantages of FDCs include ease of prescription and dose titration; reduction in the incidence of adverse events; reduction of the cost of treatment [1,2,8]. All this leads to an increase in patient compliance to treatment and therefore to increase in the number of patients achieving the target BP level as well as to reduction of the incidence of side effects.
Most drugs among FDCs are dual combinations. The most modern approach to AHT improvement is the creation and application of triple FDCs of AHDs. Triple therapy is recommended for the treatment of AH in patients whose BP is not adequately controlled by dual FDC. In this context, current clinical guidelines recommend the combination of ACE inhibitors or BRA, CCB and diuretics [1,2,8]. Most recently, in the pharmaceutical market of Ukraine a modern triple FDC was registered: valsartan-amlodipine-hydrochlorothiazide (Val+Aml+HCTZ). This triple FDC is essentially a combination of two of the most used effective dual combinations of AHDs of the last decade: ACE inhibitors or BRAs with diuretics and ACE inhibitors or BRA with CCB. Components of these FDC are the drugs of the first line in the AH treatment [1,2,8].
Analysis of the evidence of clinical effectiveness of individual components of the triple FDC Val+Aml+HCTZ confirms that these are drugs with a high level of evidence. It has been found out that thiazide and thiazide-type diuretics are preferred for the first line of AHT in patients without risk factors, superior to CCB and ACE inhibitors in the prevention of cardiovascular events (CVE) and thus less expensive [10]. High clinical efficacy of amlodipine in preventing the risk of CVE in patients with AH was confirmed in a number of multicenter clinical trials: PREVENT [11], CAMELOT [12], ASCOT-BPLA/CAFÉ [13], ALLHAT [10,14], of valsartan – in clinical trials: VALUE [15], VALIANT [16], Val-HeFT [17,18], JIKEI HEART [19], KYOTO HEART [20].
Thus, to date, there is strong evidence of clinical effectiveness of AHDs hydrochlorothiazide, amlodipine and valsartan in the reduction of the number of CVE. This was the prerequisite for the triple FDC based on them.
Clinical efficacy of the triple FDC Val+Aml+HCTZ has been proven in the randomized double-blind trial [21]. First of all, this FDC is effective and safe for the treatment of patients, uncontrolled BP with two AHDs, as well as for patients who have already received three drugs to control BP. However, to date there is no information about the pharmacoeconomic benefits of this FDC taking into account peculiarities of the Ukrainian pharmaceutical market of AHDs.
The objectiveof this research isthe pharmacoeconomic study of advantages of the new triple FDC valsartan-amlodipine-hydrochlorothiazide compared with three other AHT regimens using dual FDCs: valsartan-hydrochlorothiazide (Val+HCTZ), valsartan-amlodipine (Val+Aml) and amlodipine-hydrochlorothiazide (Aml+ HCTZ) in terms of a Ukrainian payer.
To implement this objective it was necessary to conduct:
- evaluation of AHD consumption with allocation of the share of FDCs of AHDs consumption in the pharmaceutical market of Ukraine using АТС/DDD-methodology;
- analysis of clinical efficacy of the triple FDC valsartan-amlodipine-hydrochlorothiazide according to clinical trial [21];
- determine costs and pharmacoeconomic indicators of the analyzed AHT regimens using FDCs.
Materials and Methods
Estimation of AHD consumption with allocation of the share of FDCs consumption in the pharmaceutical market of Ukraine during 2012 was carried out according to the data retrieval system MORION using ATC/DDD-methodology [21]. For pharmacoeconomic evaluation of the triple FDC Val+Aml+HCTZ the cost-effectiveness analysis was used. Cost-effectiveness ratio (CER) for each treatment regimen was calculated according to the formula (1): CER = DC/Ef (1), where DC – direct costs (costs of treatment regimen); Ef - effectiveness of treatment regimen. The costs and consequences of treatment regimens were compared in terms of the additional costs, which a treatment regimen imposes over another treatment, compared with the additional effectiveness (in terms of outcome) it provides. An incremental cost effectiveness ratio (ICER) was computed according to the formula (2): ICER = (DCr) - (DCc)/(Efr) - (Efc) (2), where is DCr - direct costs of reference treatment regimen; DCc - direct costs of compared treatment regimen; Efr - effectiveness of reference treatment regimen; Efc - effectiveness of compared treatment regimen [22].
For calculating the costs, retail prices of trade original drugs, relevant to INN FDC according to data in March 2013, were used. To convert hryvnia to euro, 13.97:1 ratio as at March 18, 2014 was used.
Results
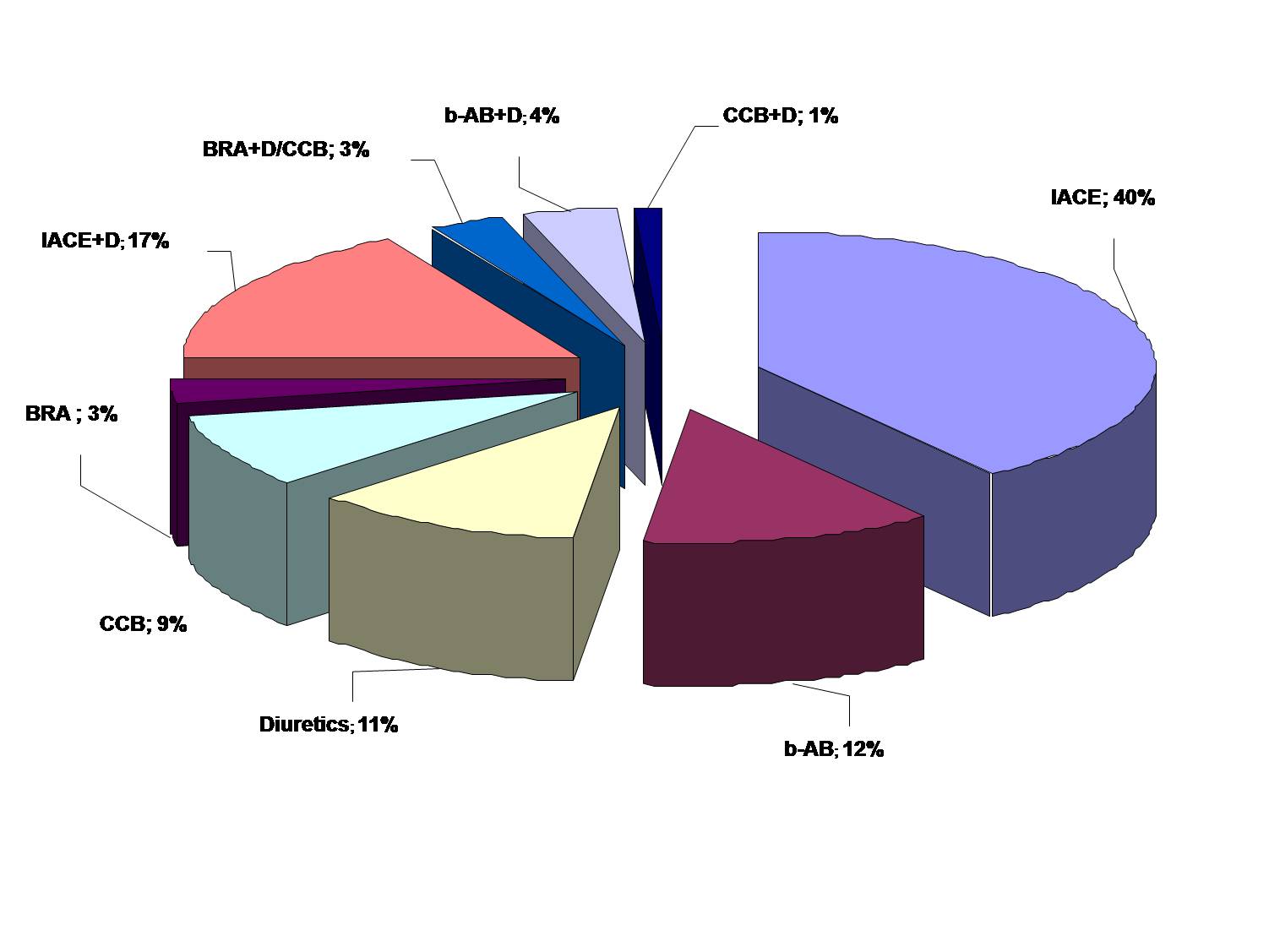
The results of evaluation of AHDs consumption in the pharmaceutical market of Ukraine during 2012 are shown in Fig. 1.
Figure 1. Structure of AHD consumption in Ukraine during 2012
(data are presented in % of the overall index DDDs/1000/d)
Note: IACE – inhibitors of angiotensin converting enzyme; b-AB - b-adrenoblockers; D – diuretics; CCB – calcium channel blockers, BRA – angiotensin receptors blockers, IACE+D – fixed combination of angiotensin converting enzyme inhibitors with diuretics; BRA+D/CCB - fixed combination of inhibitors of angiotensin receptors blockers with diuretics or calcium channel blockers; b-AB+D - fixed combination of b-adrenoblockers with diuretics; CCB+D- fixed combination of calcium channel blockers with diuretics.
The findings confirm that in the overall structure of consumption the share FDCs of AHDs is 25%. Given the high proportion (over 60%) of Ukrainian consumers (patients with AH) requiring combined AHT [2], such consumption of FDCs AHDs is not high enough to ensure effective AHT in Ukraine. This in turn indirectly indicates low compliance of patients with AH, and the need to confirm the pharmacoeconomic benefits of FDCs AHDs, in particular FDCs of a new generation the cost of packing of which is usually higher than that of monotherapies.
Evaluation of the clinical efficacy and safety of AHT using the triple FDC Val+Aml+HCTZ in patients with moderate or severe stage of hypertension (BP: systolic > 145 mm Hg, diastolic > 100 mm Hg) compared with three other AHT regimens using dual FDC: valsartan-hydrochlorothiazide (Val+HCTZ), valsartan-amlodipine (Val+Aml) and amlodipine-hydrochlorothiazide (Aml+HCTZ) was carried out according to the trial: Triple antihypertensive therapy with amlodipine, valsartan and hydrochlorothiazide: a randomized clinical trial [21].
Analysis of the clinical efficacy of the AHT regimens under study. The clinical trial [21] was carried out during 8 weeks. All the patients were divided with the application of randomization into 4 groups, the patients of which received the AHT appropriate regimen (Table 1). The patient of the first group (1st regimen) received the dual FDC Val+HCTZ at a dose of 160 mg/12.5 mg during the first week, during the next week – the triple FDC Val+Aml+HCTZ at a dose of 160 mg/5mg/12.5 mg, and during the next six months – this FDC at a higher dose of 320 mg/10mg/25 mg. The patient of the second group (2nd regimen) received the dual FDC Val+HCTZ during the first two weeks at a dose of 160 mg/12.5 mg, during the next six month – at a dose of 320 mg/25 mg. The patients of the third group (3rd regimen) received the dual FDC Val+Aml during the first two weeks at a dose of 160 mg/5 mg, during six month – at a dose of 320 mg/10 mg. The patients of the fourth group (4th regimen) received the dual FDC Aml+HCTZ during the first two weeks at a dose of 5 mg/12.5 mg, during the next six weeks – at a dose of 10 mg/25 mg.
As indicators of the clinical efficacy of the AHT regimens under study, reduction of the daily SBP and DBP was used. At the end of the study in each study group the number of patients who achieved the target BP was determined (< 140/90 mm Hg) (Table 1). It has been found that the triple FDC Val+Aml+HCTZ is the most effective compared to other treatment regimens – 70.8 % of patients who achieved the target BP. Using the triple FDC Val+Aml+HCTZ for the treatment of 1000 patients makes it possible to additionally achieve the target BP in 260 patients compared with using the dual FDC Aml+HCTZ, in 225 patients compared with the usage of the dual FDC Val+Aml and 167 patients as compared to using the dual FDC Val+HCTZ.
Table1. Characteristic of the studied regimens of antihypertensive therapy and clinical efficacy
| Treatment regimens | 1st week | 2nd week | 3rd– 9th week | Ef,% |
| 1. | Valsartan +Hydrochlorothiazide,160 mg / 12.5 mg | Valsartan +Amlodipine +Hydrochlorothiazide,160 mg / 5mg / 12.5 mg | Valsartan +Amlodipine +Hydrochlorothiazide,320 mg / 10mg / 25 mg | 70.8 |
| 2. | Valsartan + Hydrochlorothiazide,160 mg / 12.5 mg | Valsartan +Hydrochlorothiazide,320 mg / 25 mg | 48.3 | |
| 3. | Valsartan + Amlodipine,160 mg / 5 mg | Valsartan +Amlodipine,320 mg / 10 mg | 54.1 | |
| 4. | Amlodipine + Hydrochlorothiazide,5 mg / 12.5 mg | Amlodipine +Hydrochlorothiazide,10 mg / 25 mg | 44.8 | |
Note: Ef - % of patients with the achieved target BP according to data of clinical trial [21].
Analysis of safety of the therapy regimens. In the clinical trial [21] the safety of the therapy regimens under study were determined by the presence of side reactions. In the course of the study, not a single case of death was found. Less than 1 % of patients experienced serious side reactions occurring with the same frequency in each study group. Most often, the patients reported side reactions such as dizziness: 1.0 %, 1.1 %, 0.4 % and 0.2 %, hypotension: 0.7 %, 1.1 %, 0 % and 0 %, peripheral edema: 0.2 %, 0 %, 0.4 % and 0.9 %, respectively to the therapy regimens that were used: Val+Aml+HCTZ, Val+HCTZ, Val+Aml and Aml+HCTZ. Therefore, the analyzed regimens were comparable in the number and severity of side reactions, which allows not taking into account the costs associated with their correction in subsequent calculations.
Thus, AHT in patients with the moderate and severe AH using the triple FDC Val+Aml+HCTZ compared to three dual FDC: Val+HCTZ, Val+Aml, Aml+HCTZ ensures the higher clinical efficacy and, meanwhile, is as safe as the treatment using the said dual regimens.
Cost analysis. When calculating the cost of the therapy regimens under study, the cost of treatment was only taken into account, based on the retail price of the packaging of the relevant drugs, the cost of a daily and a course dose.
The obtained results of the calculation of the cost of treatment are shown in Table 2.
Table 2. Costly characteristic of the studied antihypertensive therapy regimens
| No | FDC | Pack size | Retail price of the package, € | Cost of one tablet, € | Cost of treatment in the first two weeks(14 days), € | Cost of treatment in the next six weeks(42 days), € | Total cost, € |
| 1. | Val+HCTZ | tab.160 mg + 12.5 mg, No14 | 12.52 | 0.89 | 6.23(first week) | 72.24 | |
| Val+Aml+HCTZ | tab. 177.5 mg(160/5/12.5 mg), No28 | 20.35 | 0.73 | 5.11(second week) | 60.90 | ||
| 2. | Val+HCTZ | tab.160 mg + 12.5 mg, No14 | 12.52 | 0.89 | 12.46 | 74.76 | 87.22 |
| 3. | Val+Aml | tab. 5 mg + 160 mg,No 28 | 9.67 | 0.35 | 4.90 | 29.40 | 34.30 |
| 4. | Aml+HCTZ | tab. 5 mg + 12.5 mg, No 30 | 6.47 | 0.22 | 3.08 | 18.48 | 21.56 |
In order of descending of the cost of treatment the regimens under study can be arranged in the following sequence: Val+HCTZ (87.22 €) > Val+Aml+HCTZ (72.24 €) > Val+Aml (34.30 €) > Aml+HCTZ (21.56 €). The triple FDC Val+Aml+HCTZ is the cheapest only compared to the dual FDC Val+HCTZ. The usage of the dual FDC Aml+HCTZ requires the least cost under Ukrainian reality, which provides the least clinical efficacy – 44.8% of patient with the achieved target BP. This makes it possible to use this AHT regimen as a reference during the pharmacoeconomic analysis.
Comparison of the cost-effectiveness ratio (CER) of the analyzed AHT regimens showed that the lowest cost of the efficiency unit is characteristic of the dual FDC Aml+HCTZ, but this scheme is the least efficient (Table 3).
Table3. The results of the cost-effectiveness analysis of antihypertensive therapy using fixed-dose combinations
| No | Treatmentregimen | Total cost, € | Ef | СER,€ / 1 of a patient with the target BP | Cost difference,€ | Ef add. | IСER,€ /1 add. of a patient with the target BP |
| 1. | Val+Aml+HCTZ | 72.24 | 70.8 | 102.03 | 53.90 | 26 | 2.07 |
| 2. | Val + HCTZ | 87.22 | 48.3 | 180.58 | 132.45 | 3.5 | 37.84 |
| 3. | Val+Aml | 34.30 | 54.1 | 63.40 | 15.27 | 9.3 | 1.64 |
| 4. | Aml+HCTZ* | 21.56 | 44.8 | 48.13 | - | - | - |
Note: 1) Ef – % of patients with the achieved target BP;2) Ef add. – % of patients with the achieved target BP compared with the reference therapy (Aml+HCTZ); 3) * - reference treatment regimen.
The use of dual FDC Val+Aml has the advantages of cost-effectiveness compared with the dual FDC Val+HCTZ and the triple FDC Val+Aml+HCTZ, but inferior to these regimes in terms of clinical efficacy. The triple FDC Val+Aml+HCTZ is characterized by high clinical efficiency - the proportion of patients with the achieved target BP equals to 70.8 %. When comparing the two AHT regimens: the dual FDC Val+HCTZ and the triple FDC Val+Aml+HCTZ, the latter is dominant, that is cheaper and more efficient and has greater cost effectiveness (102.03 € per 1 patient with a target BP) compared with the regimen Val+HCTZ (180.58 € per 1 patient with a target BP).
The results of the pharmacoeconomic cost-effectiveness analysis by the results of the clinical study have shown that AHT based on the triple FDC Val+Aml+HCTZ in patients with moderate and severe AH provides greater clinical efficacy compared with the other three treatment regimens using the dual FDCs and has pharmacoeconomic advantages compared with only one dual FDC Val+HCTZ.
Discussion and Conclusions
A key factor contributing to poor BP control is nonadherence to prescribed antihypertensive medications. Improving patient adherence to AHT is the key to improving BP goal attainment. For most patients, however, combinations of two or more AHDs are necessary for adequate BP control. Patient adherence to AHT decreases with increasing number of pills in multiple pill regimens, but fixed-dose triple-combination treatments for hypertension provide a tool for addressing patient nonadherence associated with pill burden. For patients whose AHT includes multiple medications, the use of a single-pill, FDC therapy can signicantly improve compliance and thereby help patients achieve BP goals [24].
Numerous single-pill, 2-drug combinations are available in the pharmaceutical market of Ukraine, and single-pill triple-combination Val+Aml+HCTZ recently received Ukrainian national authority approval. The use of single-pill, fixed-dose triple-combination therapy are appropriate in patients with uncontrolled hypertension who are taking 2 separate drugs, a 2-drug combination, or 3 separate drugs [25, 26, 27]. Prescription drug costs sometimes (but not always) are higher for single-pill combination therapies compared with the component drugs [28], yet reduced health care utilization in patients prescribed single-pill combinations.
The share of consumption (in terms of DDDs/1000/d) of FDC AHD in Ukraine during 2013 year in the total structure of AHDs consumption is 25%. This is more than in Russia (5%) [29] and closer to the volume of consumption in the European countries Germany (15%) and France (19%) [30]. Obviously, Ukrainian doctors follow the principal of current clinical guidelines in the treatment of hypertension.
But the share of FDCs AHDs consumption in Ukraine is significantly lower than the proportion of patients (60%), requiring the combined AHT. This indicates low compliance of Ukrainian patients to AH treatment and the need for pharmacoeconomic study of benefits of antihypertensive therapy using FDCs of AHDs.
The FDC of Val+Aml+HCTZ is a valuable addition to the armamentarium of drugs in the treatment of hypertension, because of its high efficacy in reducing BP, its tolerability, and the high compliance of patients with treatment. The results of the pharmacoeconomic cost-effectiveness analysis showed that AHT in patients with moderate and severe AH using the triple FDC Val+Aml+HCTZ compared to three dual FDC: Val+HCTZ, Val+Aml and Aml+HCTZ provides greater clinical efficacy (the number of patients with the achieved target BP). The said triple FDC Val+Aml+HCTZ has pharmacoeconomic advantages only compared to one dual FDC Val+HCTZ which makes it possible to save money and additional benefits of efficiency as well as justifies the advantages of its use by hypertensive patients in need of appointing the third AHD CCB amlodipine in addition to the existing dual therapy with valsartan and diuretic hydrochlorothiazide.
- Shalnova S. A., Balanova Yu. А., Konstantinov V. V. et al. Arterial hypertension: prevalence, awareness, administration of antihypertensive drugs and treatment efficacy among the population of the Russian Federation. Russian cardiological journal. 2006; 4: 45-50
- Arterial hypertension. Updated and adapted clinical guidelines based on evidence. – 2012. – 129 p. – Available from: http://www.dec.gov.ua/mtd/_ag.html
- Cohn J.N., Tognoni G., for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure N. Engl. J. Med. 2001; 345 (23): 1667-1675
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative R. search Group. Major outcomes in high–risk hypertensive patients randomized to angiotensin–converting enzyme inhibitor of calcium channel blocker vs diuretic: The antihypertensive and Lipid– Lowering treatment to prevent Heart Attack Trial (ALLHAT). JAMA. 2002; 288(23): 2981– 2997
- Dahlöf B., Devereux R. B., Kjeldsen S. E. et al. Cardiovascular morbidity and mortality in the Losartan Intervention For End point reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002; 359(9311): 995–1003
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991; 265: 3255-3264
- Hansson L., Zanchetti A., Carruthers S.G. et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension; principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998; 351: 1755-1762
- Mancia G., Fagard R., Narkiewicz K. et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) 2013. European Heart Journal. 2013. Available from: http://eurheartj.oxfordjournals.org/
- Mourad J. J., Waeber B., Zannad F. et al. Comparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approach. J Hypertens 2004; 22: 2379-2386
- The ALLHAT investigators. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002; 288 (23): 2981–2997
- Bertram P., Byington R., Curt D. et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Circulation. 2000;102:1503-1510
- Nissen S. E., Tuzcu E. M., Libby P. et al. The CAMELOT/NORMALISE study. JAMA. 2004; 18: 2217–2226
- Dahlöf B., Sever P. S., Poulter N. R. et al. Prevention of cardiovascular events with an anti-hypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendr of lumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm (ASCOT–BPLA): a multicentre randomised controlled trial. Lancet. 2005; 366: 895–906
- Frans H.H. Leenen, Chuke E. Nwachuku, Henry R. Black et al. Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2006; 48: 374-384
- Julius S., Kjeldsen S. E., Weber M. et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004; 363: 2022–2031
- Pfeffer M. A., McMurray J. J., Velazquez E. J. et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003; 349: 1893–906
- Wong M., Staszewsky L., Latini R. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002; 40: 970–5
- Maggioni A. P., Latini R., Carson P. E. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val–HeFT). Am Heart J. 2005;149: 548–57
- Mochizuki S., Dahlöf B., Shimizu M. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007; 369: 1431–1439
- Sawada T., Yamada H., Dahlöf B., Matsubara H. Effects of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART. Eur Heart J. 2009; 30: 2461–2469
- Calhoun D. A., Lacourciere Y., Chiang T. Y., Glazer R. D. Triple antihypertensive therapy with amlodipine, valsartan and hydrochlorothiazide: a randomized clinical trial. Hypertension. 2009; 54: 32-39
- Anatomical therapeutic Chemical (ATC) classification index including defined daily doses (DDDs) for plain substances. WHO Collaborating Centre for Drug Statistics Methodology. 1999: 23–33
- Vorobyov P. A. Economic evaluation of the effectiveness of drug therapy (pharmacoeconomic analysis). Moscow 2000; 80.
- Ajay K. Gupta, Shazia Arshad, Neil R. Poulter. Compliance, Safety, and Effectiveness of Fixed-Dose Combinations of Antihypertensive Agents: A Meta-Analysis. Hypertension. 2010; 55: 399-407
- Elijovich F., Laffer C. A role for single-pill triple therapy in hypertension. Ther Adv Cardiovasc Dis. 2009; 3: 231–240
- Gradman A. H. Rationale for triple-combination therapy for management of high blood pressure. J Clin Hypertens (Greenwich). 2010a; 12: 869–878
- Gradman A. H., Basile J. N., Carter B. L. et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010b; 4: 42–50
- Brixner D. I., Jackson K. C., Sheng X. et al. Assessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide retrospective cohorts in free and fixed-dose combinations. Curr Med Res Opin. 2008; 24: 2597–2607
- Leonovа M. V., Belousov D. Yu., Steinberg L. L. et al. Results pharmacoepidemiological study of hypertension PIFAGOR III. System hypertension. 2010; 1: 8-15
- Fretheim A., Oxman A. D. International variation in prescribing antihypertensive drugs: Its extent and possible explanations. BMC Health Services Research. 2005; 5: 21–30