Implications of economic crisis on health care decision-making in Hungary: An opportunity to change?
-
Copyright
© 2012 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Methods: The pricing and reimbursement process of new health technologies was reviewed to assess the transparency of decision-making and the availability of objective and verifiable criteria for reimbursement. In addition, a longitudinal analysis of public health care and pharmaceutical expenditures was conducted in Hungary between 1993 and 2011.
Results: Health policy and major reimbursement decisions are still not fully transparent and made without objective and verifiable criteria in Hungary. The Hungarian National Health Insurance Fund had continuous deficit since its foundation between 1993-2006. During this period the actual public pharmaceutical spending was higher than the planned budget. The highest overspending percentage ((actual – planned)/planned) was observed in parliamentary election years, 21.5% in 1994, 32.1% in 1998, 36.6% in 2002 and 30.4% in 2006. Since 2007 serious cost containment measures have been implemented.
Conclusion: There is still room to enforce the cost-effectiveness criterion in pricing and reimbursement decisions, as it improves the allocative efficiency of scarce public resources. The economic crisis creates an opportunity to strengthen the evidence base of health care decision making in Hungary.
Introduction
Scarcity of public resources, especially in challenging economic times, draws attention to the expenditure on health care. Pharmaceutical expenditures gained remarkable attention, as drugs are considered a major growth driver of health care spending [1,2]. Middle income countries tend to spend higher proportion of their health care expenditure on pharmaceuticals compared to developed countries: they have to purchase innovative drugs at the same global price as high income countries due to manufacturers' response to international price referencing and parallel trade, whilst their manpower costs are lower [3]. Macroeconomists (e.g. at IMF or EU) pay attention to public pharmaceutical expenditures, however, their macro-level policy recommendations usually focus solely on cost-containment, and so do not consider implications on health outcomes.
Incorporation of health economics and health technology assessment (HTA) in healthcare decision making happened earlier in Hungary than in other Central Eastern European middle income countries. In the mid 90s the World Bank supported the establishment of two new academic centres in public health and health care management. The number of trained professionals was sufficient to set up HTA & health economic centres in Hungarian universities for academic research, graduate and postgraduate training. Methodological guidelines for economic evaluations were published in 2002 [4]. Cost-effectiveness evidence prior to the reimbursement of pharmaceuticals and medical devices has become mandatory in Hungary since 2003 and 2011, respectively. In 2004 the Ministry of Health established its public HTA Office for the critical appraisal of HTA chapters in the reimbursement applications submitted by pharmaceutical manufacturers. However, documents about the evidence base of new technologies and summary report of reimbursement decisions are not routinely available for public revision or scientific research as opposed to many other countries with fourth hurdle [5,6]. Therefore is still room to improve the transparency [7].
Hungary has a single-payer health insurance system. The National Health Insurance Fund (NHIF) had continuous deficit between 1993-2006 [8]. The deficit varied from 3.4% to 12.6% between 1994 and 2002, and increased strikingly between 2003 and 2005, by reaching 31.2% in 2005 (375.3 billion HUF) [9] (1 EUR=248.05 HUF, average exchange rate in 2005). As a consequence, in the end of 2006 strict cost containment measures were implemented. The aim of this paper is to explore the implications of economic crisis on macro level health care decision making in Hungary.
Materials and methods
The process of pharmaceutical pricing and reimbursement decision was assessed with special focus on the timeliness of decisions, the transparency of decision-making process and the availability of objective and verifiable criteria for reimbursement. In addition we completed a longitudinal analysis of public health care and public pharmaceutical expenditure in Hungary between 1993 and 2011 based on NHIF data.
Results
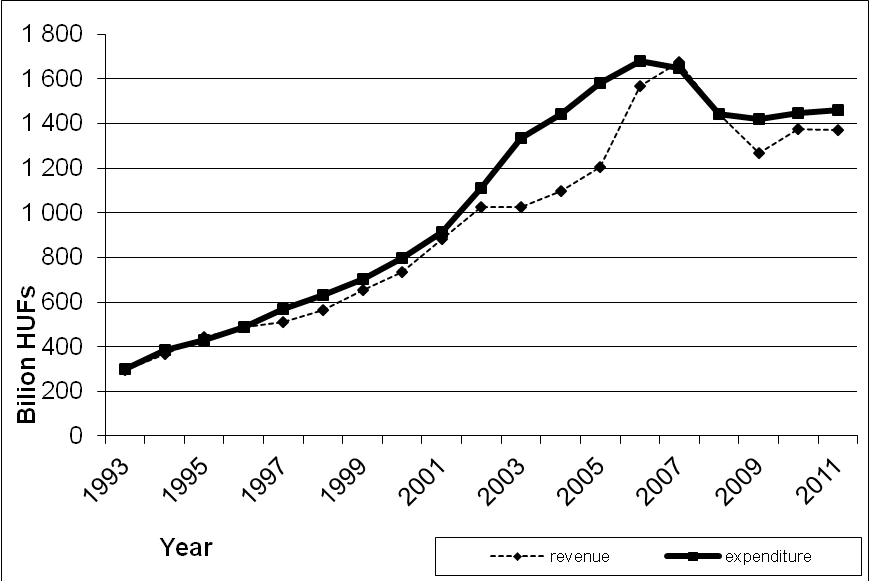
29 official resolutions of pricing and reimbursement decisions by the NHIF were analyzed between January and June 2008. In 14 cases the NHIF granted reimbursement, in 15 cases the reimbursement claim was rejected. The average time period for pricing and reimbursement procedure between the submission of the reimbursement dossier and the official decision was 172 days (minimum 43 days; maximum 534 days). As the analysis excluded those pricing and reimbursement applications with no decision, these estimates are conservative. Applications waiting for the decision over longer periods could significantly increase the time scale of pricing and reimbursement decisions. Still, several pricing and reimbursement applications with over 180 days of evaluation period were observed. In addition no objective and verifiable criteria in the pricing and reimbursement resolutions of innovative pharmaceuticals could be justified. These factors indicate serious problems with the timeliness, transparency and consistency of pharmaceutical pricing and reimbursement decisions. Figure 1 indicates the revenues and expenditures of the Health Insurance Fund in Hungary. After the 31.2% deficit in 2005, the expenditure has been considerably reduced since 2007.
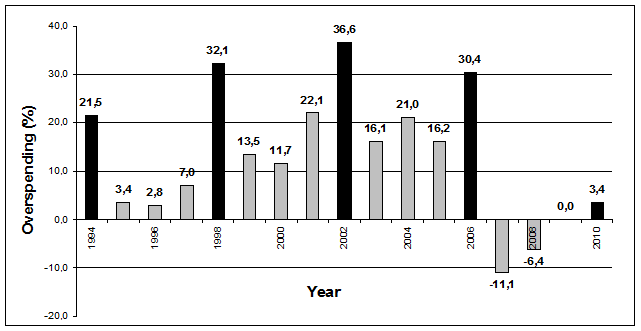
Figure 2 depicts the overspending of the Hungarian public pharmaceutical budget. Between 1994-2006 the actual public pharmaceutical spending was higher than the planned budget in each year. The highest overspending percentage ((actual – planned)/planned) was observed periodically, i.e. 21.5% in 1994, 32.1% in 1998, 36.6% in 2002 and 30.4% in 2006. Since 2007 serious cost containment measures have been implemented, in 2007 and 2008 even net savings were realised in the public pharmaceutical budget. Since 2011 the Hungarian government has introduced further cost-containment measures for the public pharmaceutical spending between 2012-2014. According to the Széll Kálmán plan, the public pharmaceutical spending has to be reduced by more than 35% in 3 years.
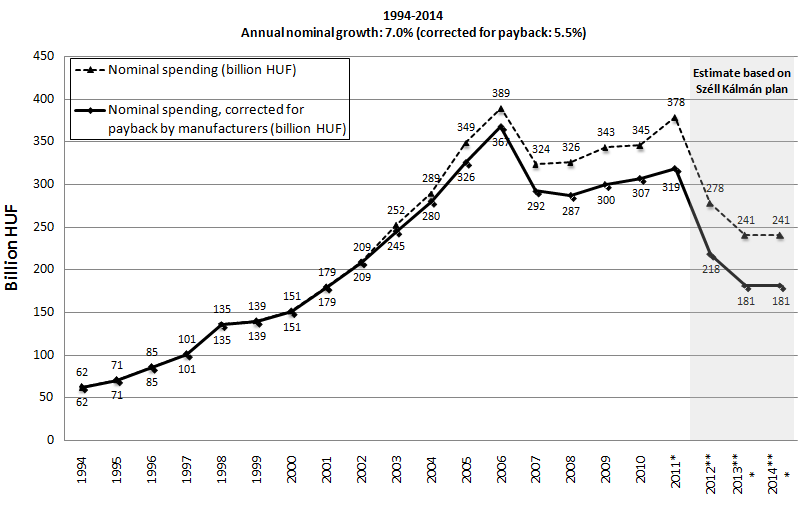
The annual growth rate of nominal pharmaceutical public expenditure was considerable until 2006 (see Figure 3). After the implementation of cost-containment measures, the public pharmaceutical expenditure decreased in 2007 and remained constant between 2008-2010. Different forms of payback mechanisms for pharmaceutical companies (including general clawback and risk-sharing agreements) have been introduced since 2003. In 2011 pharmaceutical companies already covered 15.8% of public pharmaceutical spending in Hungary, therefore the actual public spending is significantly less than what is presented in annual reports.
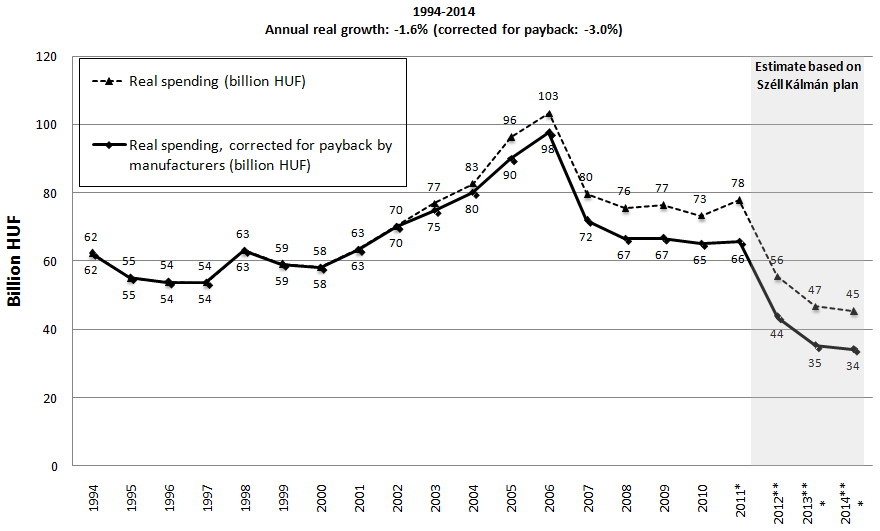
If we correct for inflation (see Figure 4), the real growth of pharmaceutical spending showed increase between 2000-2006, and significant decrease between 2006 and 2008. Figure 3 and 4 also depict the implications of the Széll Kálmán plan. The Hungarian public pharmaceutical budget will be reduced by 100.5 billion HUF in 2012 and by additional 37 billion HUF in 2013 and 2014 [10]. As part of this, reduction will be transferred to other channels of financing pharmaceuticals (i.e. approximately 30 billion HUF is allocated for central NHIF tender for special high cost drugs), the net impact of Széll Kálmán plan could be less dramatic than indicated on Figure 3 and 4.
Discussion
Figure 1 and Figure 3 indicate similar trends, and therefore confirms that pharmaceutical expenditure played significant role in the growth rate of public health care spending in Hungary. Figure 2 indicates that peaks of overspending the public pharmaceutical spending correlate with the 4-year parliamentary elections (1994, 1998, 2002, and 2006).
Figure 1, 3 and 4 reflect the serious cost containment measures implemented in the financing of health care services and pharmaceuticals after 2006 [11]. The implementation of the Széll Kálmán plan would result in negative annual real growth (-1.6%) rate within a 20-year period from 1994 to 2014 according to Figure 4 (-3.0% if real spending is corrected by payback by pharmaceutical companies). If the Széll Kálmán plan is fully implemented, the actual public pharmaceutical spending corrected by the contribution by pharmaceutical manufacturers would be 45% lower in 2014 compared to 1994. This reduction may shift additional financial burden of pharmaceutical spending to private households. As a consequence of cost-containment measures after 2006 the Hungarian health care sector is currently seriously underresourced and close to collapse. By 2011 hospitals had cumulated a huge deficit. Despite significant reduction of acute care beds, only a few hospitals were closed. Consequently the economies of scale and scope of hospitals were decreased.
The low salary of Hungarian health care professionals has been escalated to a human resource crisis. Many young physicians and nurses left the country in recent years and moved to Western Europe for significantly higher salary and career opportunities. There is a constant shortage of primary care physicians and specialists (e.g. anaesthesiologists, pathologists, traumatologists). The average age of primary care physicians is over 62 years, and many GP practices are vacant, especially in the countryside. Hundreds of young resident physicians planed to hand in their resignations.
In such a difficult economic period it is easier to justify the implementation of evidence based health policy. Consideration of cost-effectiveness evidence prior to major policy and reimbursement decisions would be essential to improve the allocative efficiency of health care financing, especially when public resources are highly limited. The authors believe that economic crisis creates an opportunity to strengthen the evidence base of decision making in Hungary, especially since there is a strong organisational structure of health economics and health technology assessment.
However, since 2006 budget impact has been the main focus of policy and reimbursement decisions, and no objective and verifiable criteria in reimbursement resolutions of innovative pharmaceuticals could be justified. Limited transparency of processes and decisions is currently one of the most critical issues in the Hungarian health care decision making. Although we could analyze only those cases with resolution (as there are several open cases without resolution for years), the time period for pricing and reimbursement decision of pharmaceuticals was still longer in several cases than the 90 + 90 days recommended by transparency directive of the European Union [12].
Conclusion
Hungary has sufficient human resource capacity and initial experience to implement evidence based health care financing and health policy. However, reimbursement and policy decisions are still not fully transparent, and as a consequence of the economic crisis, the emphasis is currently on cost-containment, i.e. budget impact instead of cost-effectiveness analysis. The most critical question for policy-makers is whether they really want to improve the rationale of health care decision making or they should just concentrate on reducing the public health care spending, as experienced in recent years. Implementation of evidence based health policy is more complicated route in the short-term, but it may pay off in the long-term.