Cost-effectiveness analysis of methods of diagnosis of tb infection in russian regions by use of recombinant tb allergen in children and adolescents.
-
Copyright
© 2017 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Background. Russia is among the countries with a high burden of TB. A new diagnostic method for TB has been developed, called ‘recombinant tuberculosis allergen’ or RTA (Diaskintest®), which has a similar cost and method of administration to the Mantoux test, but the principle of action, sensitivity and specificity are similar to IGRA technology. Despite the improvements of RTA, TB experts continue to use the Mantoux test. The aim of this study was to determine the most cost-effective medical technology for diagnosing tuberculosis infection in the Russian Federation, basing on calculation of ICER.
Methods. The study was based on 3 models simulating the diagnosis of TB in Russia: 1-Mantoux test and further diagnostics methods (based on screening in 2008 in 1,302,490 Moscow children and adolescents); 2-Mantoux test with subsequent use of RTA in case of a positive Mantoux test, combined with further diagnostics methods (based on screening in 2013 in 1,420,100 Moscow children and adolescents); 3-RTA alone and further diagnostics methods (data based on screening conducted in 2015 in 108,729 Penza region children and adolescents 8-17 y.o.).
Results. Method effectiveness (percentage of active TB infection among screened individuals), was 0.006%, 0.012% and 0.019% for models 1, 2 and 3, respectively. Cost-effectiveness ratios were 41861.67, 18401.67 and 10512.12 for models 1, 2 and 3, respectively.
Conclusions. RTA is the most cost-effective tuberculosis screening method in children and adolescents, superior to both the stand-alone Mantoux test or the Mantoux test followed by RTA.
INTRODUCTION
Tuberculosis (TB) is a bacterial infection of humans caused by various kinds of mycobacteria from the group of Mycobacterium tuberculosis complex. The vast majority of infected individuals have asymptomatic disease (latent form), and only one in ten latent cases progress to active disease. [1] The risk of developing active tuberculosis is associated with a number of factors, the most important of which is the immune status of the subject. According to the WHO, the number of global tuberculosis cases was 9.0 million people in 2013, including about 550 000 children. The number of people with TB becoming sick is decreasing steadily, with a reduction in death rate of 45% in the period from 1990 to 2013. Despite this decline in the incidence globally, the Russian Federation saw an increase in the incidence of multidrug-resistant TB. In 2007-2012 there was a decrease of TB incidence in the Russian adult population (from 63.7 in 2007 to 52.1 per 100.000 adults) and in children (from 17.7 to 13.7 per 100.000 children). At the same time there was an increase of "bacillary nucleus" marked (infectious) with multidrug-resistant pathogen (from 15.7%, in 2007 to 22.6% in 2012) of all new cases of bacterioexcretion. Based on these data one can conclude that Russia is among the countries with a high burden of TB. [2]
Detection of the presence of Mycobacterium tuberculosis in the patient can be done only via histological examination of pathological material when tuberculosis is present. Early diagnosis of TB is problematic and often requires a multitude of medical disciplines. Important factors of diagnostic methods are their sensitivity (probability of a positive test result in individuals with disease) and specificity (probability of negative results in persons without disease). The following methods for the diagnosis of TB in a wider population are available:
1. Traditional tuberculin diagnosis. Intradermal Mantoux test with 2 TE PPD-L using a purified TB allergen in the standard dilution.Despite widespread use, there are still a number of unresolved questions regarding the specificity and sensitivity of the tuberculin test in active tuberculosis and in latent TB infection.
2. Interferon Gamma Release Assays (IGRA). The discovery of antigens specific for M. tuberculosis has led to the development of in vitro tests based on the production of interferon-γ (IFN- γ) by T-lymphocytes as a response to stimulation with tuberculosis antigens, called IGRA.
According to the recommendations of the European Society for Tuberculosis [3], a positive tuberculin reaction has a low predictive value, while a positive IGRA test has a high predictive value. This recommendation applies to countries with widespread BCG-vaccination (Bacillus Calmette-Guérin), including Russia, because cross-reactions with BCG strains often lead to false results of tuberculin skin tests. Therefore, when deciding on preventive therapy it is recommended to take into IGRA test results into account. However, the high costs of these tests, the necessity to adequately equip laboratories, the route of administration (I.V.) and the specific conditions for storing and using IFN- γ producing lymphocytes, do not allow IGRA as a method for mass diagnosis of TB infection.
A recombinant TB allergen diagnostic test (Diaskintest®) has been developed in Russia with a cost and administration similar to the Mantoux test, but the principle of action, sensitivity and specificity are similar to IGRA technology, e.g. QuantiferonTB-Gold® test. Despite numerous studies proving the efficacy, safety and efficiency of the use of the recombinant tuberculosis allergen, some TB experts still continue to use the Mantoux test in clinical practice for the diagnosis of TB infection.
OBJECTIVES
Aim of the study was to determine the most cost-effective method of diagnosis of TB in children and adolescents in the Moscow region. This was done by conducting a cost-effectiveness analysis in order to determine the cost-effectiveness ratios of available diagnostic tests for tuberculosis basing on Russian data.
MATERIAL AND METHODS
A retrospective cost-effectiveness analysis was performed by modeling the use of the recombinant tuberculosis allergen in the diagnosis of TB in the Russian Federation. Three models were built:
• Model 1: TB screening in children and adolescents (1-17 y.o.) using Mantoux testing and further diagnosis [5];
• Model 2: TB screening in children and adolescents (1-17 y.o.) using Mantoux testing with subsequent use of recombinant TB allergen in case of a positive Mantoux test [6];
• Model 3: TB screening in children and adolescents (8-17 y.o.) using recombinant TB allergen [7].
Model №1 was based on data of the Moscow City Scientific and Practical Center against TB from 2008, before the introduction of the recombinant TB allergen in clinical practice. This data thereby eliminates any possible confounding of TB allergen use on the study results. Model №1 included children and adolescents that have been screened for tuberculosis in the Moscow area in 2008 (1,302,490 subjects).
Model №2 used data from screening of children and adolescents conducted in 2013 in Moscow (1,420,100 subjects). Recombinant TB allergen was applied after using the Mantoux test in patients selected for further examination in special anti-TB institutions.
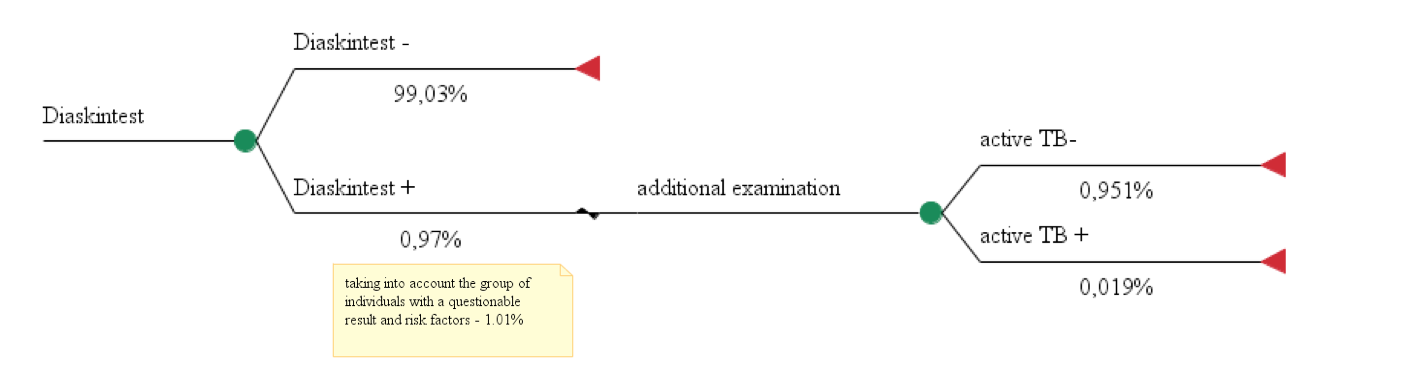
Model 3 looked at the use of the recombinant TB allergen based on research data from Stasko E.Y., Khasanshin G.S. et al. who studied the use of skin testing with recombinant TB allergen as a screening method for TB in children and adolescents 8-17 years old in 2015, which included 108,729 children. Due to the fact that the literature search did not result in any study focusing on the use of only recombinant TB allergen in screening of children and adolescents in Moscow, data was used from studies of children in other Russian regions. A survey of experts was undertaken, the results of which showed that the frequency of positive reactions to allergen recombinant TB in children and adolescents in 4 regions were as follows: 1%; 0.7%; 1.25%; 0.7%, which corresponds to the figures given in the study of E.Y. Stasko et al. In this study a computed tomography of the chest was performed (reference test) in children with a positive reaction to the recombinant tuberculosis allergen (n=1044, 0.97%), as well as children with questionable results of the test with recombinant tuberculosis allergen (n=52 children), a total of 1096 patients (1.01 %). According to the results, computed tomography identified 21 (1.9% of 1096 surveyed) children with active pulmonary TB.
RESULTS
The systematic literature review showed that tests that recombinant tuberculosis allergen tests are more effective in diagnosing TB in children and adolescents than the Mantoux test. Publications with data on costs and effectiveness of recombinant tuberculosis allergen were included in the pharmacoeconomic modeling.
Development of the model. The model contains two separate, but interlinked components: a decision tree and a detailed calculation of costs for all the studied diagnostic methods for TB in children and adolescents.
Cost analysis. For the calculation of the cost of recombinant tuberculosis allergens pricing data registered in the State Register of Limiting Selling Prices was used [4] for recombinant tuberculosis allergen as at 12/11/2015] as well as information on the cost of services related to TB diagnosis from the Moscow City Scientific and Practical Center against tuberculosis [d.d. 25/01/2016]. The costs for the purchase of the Mantoux test with 2 TE PPD-L was derived from information from the “St. Petersburg City polyclinic №106”. [5] The costs of the different diagnostic methods are presented in Table 1.
Table 1. Costs of TB screening
|
|
Diagnostic method |
Cost (€/patient) |
|
1. |
Mantoux test |
1,22 |
|
2. |
recombinant tuberculosis allergen test (Diaskintest) |
1,28 |
|
3. |
TB specialist primary admission |
7,60 |
|
4. |
TB specialist seondary admission |
6,08 |
|
5. |
Panoramic X-ray |
3,73 |
|
6. |
General blood analysis |
7,19 |
|
7. |
General urine analysis |
3,96 |
|
8. |
CT scan of the chest |
48,54 |
|
9. |
Additional examination (includes 4 - 8) |
69,5 |
Effectiveness criteria. The most important effectiveness criteria used in the diagnosis of active TB are specificity, sensitivity and the number of diagnosed patients with active TB. In the clinical trials identified in this study, the specificity and sensitivity of the recombinant TB allergen used in TB diagnosis in children and adolescents was higher than the Mantoux test, including studies that confirmed diagnosis via thee reference test (computed tomography, fluorography, Quantiferon test). The purpose of this study was to compare new diagnostic methods of TB with existing methods, therefore it was decided to evaluate the effectiveness by comparing the number of diagnosed patients with active TB.
Cost-effectiveness analysis (calculation of CER, cost-effectiveness ratio). T
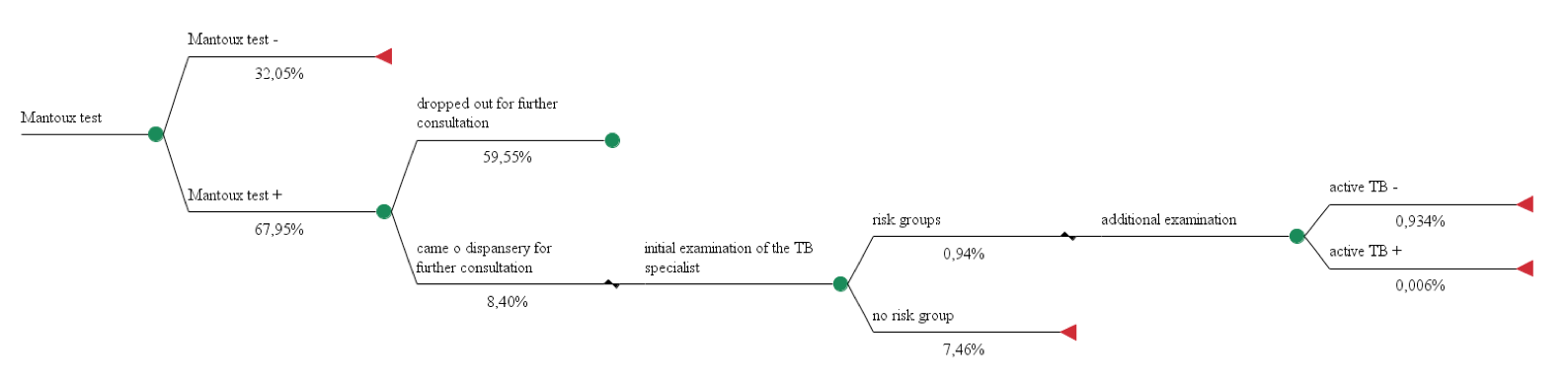
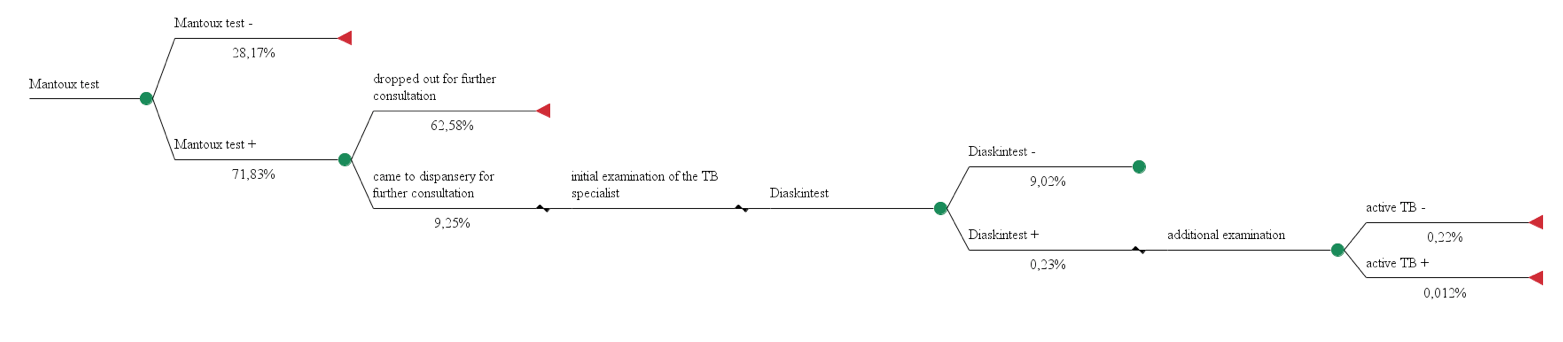
We built 3 pharmacoeconomic models using alternative approaches for diagnosis of tuberculosis in children and adolescents and calculated their costs. Cost data used in the calculation models № 1, 2, 3 are shown in Table 1, effectiveness of diagnostic techniques - in Figures 1, 2, 3.
Model 1. The use of the Mantoux test for diagnosis of active tuberculosis in 100 people.
Total costs = 1,22€*100 + 7,60€*8,40 + (48,54€ + 3,96€ + 7,19€ + 3,73€ + 6,08€) * 0,94 = 251,17€
Effectiveness = 0,006%
CER = costs of health technology / effectiveness of health technology
СЕR1 = 251,17€ / 0,006 % = 41 861,67
Model 2. The use of Mantoux test and recombinant TB allergen for diagnosis of active tuberculosis in 100 people.
Total costs = 1,22€*100 + (7,60€ + 1,28€) *9,25 + (48,54€ + 3,96€ + 7,19€ + 3,73€ + 6,08€) *0,24 = 220,82 €
Effectiveness = 0,012%
СЕR2 = 220,82 €/ 0,012% = 18 401,67
Model 3. The use of recombinant TB allergen for diagnosis of active in 100 people.
Total costs = 1,28€ *100 + (48,54€ + 3,96€ +7,19€ + 3,73€ + 7,60€) * 1,01= 199,73€ Effectiveness = 0,019%
СЕR3 = 199,73€ / 0,019%= 10 512,12
Sensitivity analysis. To assess and forecast the impact of changes in the input data to the modelling results, a sensitivity analysis was conducted taking into account the impact of minimum and maximum medicine costs, and the percentage of correctly diagnosed patients:
1. Reducing the cost of the Mantoux test from 1,22€ to 0,34€ (arbitrary reduction) leads to a reduction of costs in Models 1 and 2 versus Model 3, while the cost-effectiveness ratio for Model 3 stays the lowest compared to Models 1 and 2.
2. Increasing the costs of the recombinant TB allergen test from 1,28€ to 2,71€ (arbitrary increase) leads to increased costs in Models 2 and 3 versus Model 1. The CER for Model 3 remains lower than in Models 1 and 2.
3. Reducing the detection rate of active TB among the positive results on recombinant TB allergen from 1.9% to 1.09% (a decrease of efficiency of 0.019% to 0.011%, respectively) leads to an increase in the CER for Model 3, while still remaining lower than the ratios in Models 1 and 2.
DISCUSSION
Basing on the results of the study, it should be concluded that the use of the recombinant tuberculosis allergen in screening for tuberculosis in children and adolescents is more cost-effective when compared to the Mantoux test or a Mantoux test followed by the use of recombinant TB allergen.
There has been found one study cost-effectiveness analysis of Mantoux and recombinant tuberculosis allergen [9], in which recombinant tuberculosis allergen is also the most cost-effective method of TB screening.
It is economically feasible to use recombinant TB allergen in tuberculosis screening of children and adolescents due to the lowest cost-effectiveness ratio versus the alternatives.
Athough, there were some limitations of the modeling results:
1. Cost data were obtained at the Health Care Institution of the State Treasury "Moscow City Scientific and Practical Center against tuberculosis of the Moscow City Health Department".
2. Study population is all surveyed children and adolescents (1 - 17 years) undergoing screening.
3. In case of examination of children in polyclinic and pediatric TB departments of hospitals, as well as in regions with a different epidemiology, it is possible to obtain different results.
4. Due to the lack of studies of a larger group of children and adolescents who used recombinant TB allergen, effectiveness data in Model 3 were derived from a study population of 108.729 people, that is considerably smaller than the group of children in Models 1 and 2 (1,302,490 and 1,420,100 children, respectively).
5. Due to the lack of research in a larger group of children and adolescents who have used only recombinant TB allergen in Moscow, effectiveness data in Model 3 were taken from a study conducted in a children and adolescents 8-17 y.o. from a population in a region with possibly another epidemiological situation (Penza and the surrounding Penza region) than in the Models 1 and 2 (Moscow). There has been a expert survey in Moscow and in the regions where pilot projects are implemented with the use of the recombinant TB allergen for the diagnosis of TB (Chuvash Republic, the city of Nizhny Novgorod, Yaroslavl, Kazan) conducted. A sensitivity analysis was conducted, when the ratio of detected active tuberculosis among the positive results when using recombinant TB allergen was equal to 1.09%.
6. In the study taken as a base of the Model 3, children with questionable results test with recombinant TB allergen because of high risk factors were included in the group for the reference test, although this number was relatively small (52 out of a total of 1096 children).
7. Due to the lack of studies of children and adolescents who used recombinant TB allergen, data from studies in different time periods were used (2008, 2013 and 2015).
CONCLUSIONS
1. The use of the recombinant tuberculosis allergen in screening for tuberculosis in children and adolescents is more cost-effective when compared to the Mantoux test or a Mantoux test followed by the use of recombinant TB allergen.
2. Reducing the cost of the Mantoux test significantly does not change the results of the study.
3. Increasing the costs for the use of recombinant tuberculosis allergen to €2.71 for one test does not affect the results of the study, i.e. the use of recombinant TB allergen is still a more cost-effective technology for screening for tuberculosis in children and adolescents compared to the Mantoux test or a Mantoux test followed by use of recombinant TB allergen.
[1] Effectiveness data: Slogotskaya L.V., Ovsyankina E.S., Kochetkov, Y.A. Stakheyeva L.B. Infection with tuberculosis of children and adolescents - a look through the century. [6] Cost data: State Register of Limiting Selling Prices, http://grls.rosminzdrav.ru/ [4], http://zakupki.gov.ru/ [5].
[2] Effectiveness data: Slogotskaya L.V., Senchikhina O.Y., Nikitina G.V., Bogorodskaya E.M. The effectiveness of recombinant tuberculosis allergen skin test in detecting tuberculosis in children and adolescents in Moscow in 2013. [7] Cost data: State Register of Limiting Selling Prices, http://grls.rosminzdrav.ru/ [4], http://zakupki.gov.ru [5].
[3] Effectiveness data: Stasko Е.Y., Khasashin G.S. Analysis of the use of skin testing with recombinant tuberculosis allergen as a screening method of screening for tuberculosis children older than 7 years in the Penza region.[8] Cost data: State Register of Limiting Selling Prices, http://grls.rosminzdrav.ru/ [4] , http://zakupki.gov.ru [5].
1. Electronic resource URL: “World Health Organization Tuberculosis - fact sheet” N°104, March 2015 [cited: 11.12.2015]. Available from: http://who.int/mediacentre/factsheets/fs104/ru/.
2. Russian Ministry of Health Order № 951 of December 29, 2014 “On approval of recommendations for improving the diagnosis and treatment of pulmonary tuberculosis.”
3. Mack U., Migliori G., Sester M., et al. for TBNETCONSENSUS STATEMENT LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009; N. 33; 956–973.
4. Electronic resource URL: http://grls.rosminzdrav.ru. [cited: 11.12.2015]
5. Electronic resource URL: http://zakupki.gov.ru. [cited: 25.01.2016]
6. Slogotskaya L.V., Ovsyankina E.S., Kochetkov, Y.A. Stakheyeva L.B. Infection with tuberculosis of children and adolescents - a look through the century.Tuberculosis and lung diseases 2011; N. 3; 21-28.
7. Slogotskaya L.V., Senchikhina O.Y., Nikitina G.V., Bogorodskaya E.M. The effectiveness of recombinant tuberculosis allergen skin test in detecting tuberculosis in children and adolescents in Moscow in 2013. Pediatric pharmacology 2015; Vol 12; N. 1 ; 1-5.
8. Stasko Е.Y., Khasashin G.S. Analysis of the use of skin testing with recombinant tuberculosis allergen as a screening method of screening for tuberculosis children older than 7 years in the Penza region. Tuberculosis and lung diseases; 2016; N. 3 ; 1-5.
9. Yagudina R.I., Zinchuk I.Y. Pharmacoeconomic study of drugs used for TB screening. Pharmacoeconomics; 2013; Vol. 6, N. 1.