Development of education on Pharmacoeconomics in Ukraine
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
The education on pharmacoeconomics for postgraduate pharmacists has been introduced in Ukraine since 2000. The objects, the pharmacoeconomic methods and evidence-based pharmacy, specific terms are being systematized and adapted in Ukraine. The discipline of “Pharmacoeconomics” has been entered into curricula, programs for graduate and postgraduate students specializing in pharmacy and clinical pharmacy. We published textbook, tutorials for study on pharmacoeconomics and the Ministry of Health of Ukraine approved of the the pharmacoeconomics curricula, the first author’s textbook for students - "Principles of pharmacoeconomics" (2002), for postgraduate pharmacists “Pharmacoeconomics” (2007) used in 12 medical (pharmaceutical) universities in Ukraine.
The active conduct of scientific pharmacoeconomic research in Ukraine, their results are used in the Central Formulary Committee Health of Ukraine while reviewing the State formulary and formulary committees for local formularies. In Ukraine 3 doctoral dissertations and 21 PhD theses were defended during 2004-2012. The results of pharmacoeconomic research in budget purchase and for creation of State Formulary of medicine of Ukraine and local formularies in medical hospitals were used.
Since 2000 the World Health Organization (WHO) and the Pharmaceutical International Federation (FIP) have developed a strategic plan for the globalization of pharmaceutical education, creation of appropriate training modules pharmacist as an expert in modern health care, which provides physicians and health care managers with proven information about the cost effectiveness of medical technology and increases quality pharmaceutical care [1,3]. The analysis of the curriculum of pharmaceutical faculties in Europe showed that the pharmacoeconomics discipline was included in the curriculum of the Bachelor, Master of Pharmacy and Doctor of Philosophy [1,2]. In 2000 Pharmacoeconomics as a discipline was introduced into the curriculum and training program of postgraduate education for pharmacists in Ukraine.
The organization of the health care system in Ukraine
According to the Constitution of Ukraine every citizen has the right to receive free medical care. Financing of the health care is offered at the expense of the state budget and local budgets. Mandatory health insurance is still unavailable. Also financing health care is subject to voluntary private insurance.
In 2002, the Ministry of Health of Ukraine approved of the "Using pharmaeconomics evaluation of medicine" guidelines which contain methods of pharmacoeconomic analyses applied in practice in the provincial departments of health of 25 regions in Ukraine. These guidelines are used in planning costs for state programs providing drugs for population and these programs are aimed at oncology, diabetes patients and at victims of Chernobyl.
The registration system of side effects of drugs was introduced in Ukraine in 2006. Systematized data on identified serious side effects are submitted annually to the Ministry of Health and published in medical journals.
The Cabinet of Ministers of Ukraine adopted a resolution in 2010 which was introduced to conduct mandatory verification of medicinal products registered in Ukraine according to the requirements of good manufacturing practice (GMP).
In January 2011 Ukraine, which is represented by the State Service of Ukraine on drugs, became a member of the International System of Pharmaceutical Inspection Cooperation (PIC/S).
The Ukrainian pharmaceutical market has a register of almost 14 000 medicines of which only 10% are the original drugs. In Ukraine State Program on Population drugs was approved during 2004-2010 with the assistance of the fundamentals of the formulary system of medicine. In 2009 the state Ministry of Health approved of the first state formulary for medical hospitals. In 2012 the 4th edition of the state formulary of medicines used in medical hospitals was approved.
The leading institution which performs certain functions i.e. HTA - Health Technology Assessment are and a special department in the State Expert Center of MoH Ukraine Central Formulary Committee. This is the department of the rational pharmacotherapy and the provision of the state formulary system. The following HTA elements are used to enter a drug into the state formulary of Ukraine:
A pharmaceutical manufacturer submits documentation, which provides registration data, and shows whether the drug is entered in international clinical protocols and guidelines, WHO formulary, other formularies built on the principle of evidence, guidelines from HTA (as NICE, SIGN), systematic reviews, results of randomized clinical trials, cohort studies, case control and economic research.
In the absence of any information the formulary is not created. All materials are submitted in two copies of the cover letter and all enclosed printed materials serving as proof of international studies and published results of national pharmacoeconomic studies. The experts of the Formulary Committee conduct a preliminary review and issue an opinion on registration of the drug in the State formulary of Ukraine.
At the second stage the Committee assembles once per month. The Expert Commission consists only of clinical experts. The list of experts from the pharmaceutical manufacturer is not open to the public. It is usual that main specialists MOH are in the Commission. The same documentation is considered as in the first stage. With the positive outcome of the previous stage, they can adopt or reject or postpone a State Formulary decision until the following assembly.
The ordinance of the Ministry of Health of Ukraine No 769 of 13.09.2010 was approved under the name of “Concept of the pharmaceutical sector of Health for 2011-2020”, according to which the system of formulary drugs for the Ukranian population will be actively implemented.
There is a necessity to create an independent HTA Agency for further implementation of the formulary system. The Agency will create a database of already conducted PE research in Ukraine and inform about their results and the adequacy of the budget for medicines, in particular innovative drugs.
The creation of the HTA Agency is particularly important due to the resolution of Cabinet of Ministers of 5th September 2012 No 907 On approval of the partial reimbursement of medicines for the treatment of patients with hypertension.
The educational process on Pharmacoeconomics in Ukraine
In general methodological aspects of evidence-based pharmacy and pharmacoeconomics processed by Western scientists i.e. M.Drummond, JS.McCombs, JF.Mauskopf, K.Bonk and others [2,4]. What has been done to support the learning process of pharmacoeconomics in Ukraine? Since 1999 authors have systematized and adapted in Ukrainian the objects of pharmacoeconomics research and evidence-based pharmacy, specific terms according to the ISPOR terms.
In 2000 the collaboration with International Society for Pharmacoeconomics and Outcomes Research – ISPOR began, which is the lead agency for the coordination and development scientific and practical pharmacoeconomic research globally. As the initiative of the Department of Management and Economy and Medicine Technology, Faculty of Postgraduate Education the discipline “Pharmacoeconomics” for postgraduate education of pharmacists was introduced in 2000. As the first informational support of practical pharmacists in the periodicals pharmaceutical publications "Halician Pharmacy" (Lviv), "Weekly Pharmacy" (Kyiv) the educational materials on pharmacoeconomics were published [7].
In 2001 we created and published the first standard curriculum “Pharmacoeconomics” for students of pharmaceutical faculties, approved by the Ministry of Health of Ukraine. In 2002, the Ministry of Health of Ukraine approved the first author's textbook for students "Principles of pharmacoeconomics" in Ukranian, used in 10 medical (pharmaceutical) universities.
In 2003, the “Pharmacoeconomics” discipline was included into the curriculum of postgraduate training of pharmacists on the following specialties - "organization and management of pharmacy" and “general pharmacy”. There was a need for a new adaptation for practical continuation of education for pharmacists. We prepared a textbook for postgraduate training of pharmacists - “Pharmacoeconomics” approved by MoH in 2007. This tutorial contains the requirements already enforced Presidential Decrees, Resolutions of the Cabinet of Ministers, orders of MoH regulating the use of pharmacoeconomic analysis in the development of formulary for medical hospitals as well as the creation of the State Formulary of medicine in Ukraine.
In methodological developments of practical training of pharmacoeconomic analysis materials for problem-based learning on specific local data were prepared control tests about pharmacoeconomic evaluation of hypolipidemic drugs based on evidence-based data concern therapeutic efficacy, effectiveness, hypoglycemic agents in patients with diabetes mellitus were included. These control tests include the availability of the domestic product, its dosage , packaging, wholesale cost of the producer.
Subsequent tutorials on pharmacoeconomics in Ukrainian scientists distributed to other educational institutions. It should be noted that there is a significant differentiation of teaching pharmacoeconomics material for pharmacists studying "organization and management of pharmacy". They pay more attention to issues of formulary supply of patients determining the need for medications. Pharmacist stuing "general pharmacy" obtain more information on pharmacoeconomic aspects of pharmacotherapy of common diseases to apply knowledge related to patient-based recommendations for effective, safe and cost-effective pharmacotherapy. At practical course and seminars postgraduate students acquire skills of pharmacoeconomic evaluation of drugs using information from a database of evidence-based medicine, computed cost-effectiveness ratio of drugs to treat common diseases. We have conducted educational courses for 6000 practical pharmacists in the Western regions of Ukraine.
In 2007, according to the requirements of Bologna educational process, in collaboration with scientists from the National Pharmaceutical University (Kharkiv) we developed the curriculum of "Pharmacoeconomics" for credit-modular system approved by the Ministry of Health of Ukraine. We published in Ukraine the first textbook "Pharmacoeconomics" (author O.Zaliska, ed.B.L.Parnovsky. - 2007. - 376 p.), which is approved by the Ministry of Health of Ukraine for training students of faculties such as “Pharmacy” and “Clinical Pharmacy” [8,9]. The curriculum and the textbook are used to educate pharmacists and clinical pharmacists in 10 medical universities (Table 1).
Table 1. Medical universities which offer training on pharmacoeconomics in Ukraine
|
Educational institution |
Pharmacy/ Clinical Pharmacy |
Educational level |
|
|
Graduate |
Postgraduate |
||
|
M.Pirogov Vinnitsa National Medical University |
+/+ |
+ |
|
|
Bukovyna State Medical University (Chernivtsi) |
+/+ |
+ |
|
|
Danylo Halytsky Lviv National Medical University |
+/+ |
+ |
+ |
|
Dnipropetrovsk Medical Academy |
/+ |
+ |
|
|
Donets State Medical University |
+/ |
+ |
|
|
Ivano-Frankivsk National Medical University |
+/ |
||
|
Lugansk State Medical University |
+/+ |
+ |
|
|
O.Bohomolets National Medical University (Kiyv) |
+/ |
+ |
|
|
P.Shupyka National Academy of Postgraduate education (Kiyv) |
+/ |
+ |
|
|
National Pharmaceutical University (Kharkiv) |
+/+ |
+ |
_ |
|
I.Horbachevsky Ternopil State Medical University |
+/+ |
+ |
|
|
Kharkiv Medical Academy of Postgraduate education |
+ |
+ |
|
|
Zaporizhya State Medical University |
+/+ |
+ |
+ |
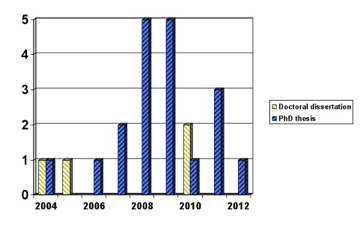
In 2004 Olha Zaliska successfully defended the first doctoral dissertation “The theoretical basis of “Pharmacoeconomics“ and its practical use in Ukraine”, which gave a strong impetus for the development of scientific and pharmacoeconomic studies, the results of which were implemented in practice of medical and pharmaceutical [5]. Now are conducted research pharmacoeconomic studies, these results are presented and defended 3 doctoral and 21 PhD theses in Ukraine. The dynamics of pharmacoeconomics is research presented in Figure 1.
In 2001, at the ISPOR invitation Olha Zaliska made an podium presentation at the 4th ISPOR European Congress in Cannes (France), which showed the first steps of study as a discipline of "pharmacoeconomics" in the education process and health care in Ukraine, contributed to the development of educational technologies in Ukraine.
In January 2008 based on results of training of pharmacoeconomics in Ukraine, ISPOR Board approved of the Ukrainian chapter - USPOR at the Danylo Halytsky Lviv National Medical University and ammended the English page on website with www.ispor.org/local_chapter/Ukraine.
Key activities of Ukrainian USPOR include processing techniques of pharmacoeconomic studies according to international requirements and recommendations relevant to national health care, use of their results at the legislative level, particularly when viewing the National List of essential medicines and medical devices which is the basis for public procurement. Priority directions of the Ukrainian Department USPOR are to train health professionals with the knowledge of pharmacoeconomic methodology and its terms.
To implement educational and practical directions on pharmacoeconomics on 17April, 2008 at the Department of Organization and Economy of Pharmacy and Medicine Technology Faculty of Continuing Education of Danylo Halytsky Lviv National Medical University a "Pharmacoeconomics in Ukraine” conference was held: status and prospects of pharmacoeconomic studies" conducted by scientists of the department and with the participation of the heads of pharmacy, specialists in organizing and managing pharmacy in Lviv and Lviv regional pharmaceutical corporations [6].
For a more extensive review of the activities of Ukrainian pharmacoeconomic studies (USPOR) we have created the first Ukrainian website online www.uspor.org, which contains information materials from international meetings and congresses on pharmacoeconomics, bibliographic sources of pharmacoeconomic studies published in national and international journals. We also consider the possibility of a web page teaching materials on the pharmacoeconomic analysis for e-learning practice and future pharmacists and their quality of methods in pharmacoeconomics.
The leading aim of pharmacoeconomics Ukrainian website is to spread knowledge of the science, pharmacoeconomics methods among professionals of medicine and pharmacy, on current trends in pharmacoeconomic studies according to the International Society of pharmacoeconomic studies - ISPOR and presentation of results at national conference and ISPOR European Congresses.
During the training courses pharmacists learn the history of pharmacoeconomics, its objects, object studies, relationships with other pharmaceutical, medical sciences, have a detailed study time in accordance with the requirements of the International Society of pharmacoeconomic research (ISPOR) from the official publication " ISPOR Book of Terms". Students get acquainted with the methods of pharmacoeconomic analysis of drugs "cost-effectiveness", "minimizing cost", "cost-benefit", "cost-benefit", "cost of illness" and use their results to optimize pharmacotherapy. Much attention is devoted to the principles of drug formulary system software. It is in accordance with the requirements of “Concept development of pharmacy sector for 2011-2020” which involves the use of pharmacoeconomics and the formulary system in Ukraine.
It should be noted that the differentiation is made in teaching materials in pharmacoeconomics: in the faculty of "organization and management of pharmacy" greater attention is paid to the organization of the formulary of patients determining the need for medications. Pharmacist faculty of "general pharmacy" focus more information on pharmacoeconomic aspects of pharmacotherapy of common diseases for further application of knowledge to provide patient-based recommendations for effective and cost-effective drug.
In practical seminars the focus is the evaluation of cost-effectiveness ratio calculated for drugs to treat common diseases.
By the decision of the International Society for Pharmacoeconomic Research (ISPOR) (2008) Ukrainian Chapter of the ISPOR (USPOR) was established. The Ukrainian website www.uspor.org was created. It presents information materials on the activities of international organizations and educational materials, research papers on theoretical directions and results of pharmacoeconomic studies in Ukraine.
Since 2008 the Department of Management and Economy of Pharmacy and Medicine Technology Postgraduate Faculty has been systematically conducting scientific and practical conferences for pharmacists on "Pharmacoeconomics globally and Ukraine", which deals with topical messages of pharmacoeconomic studies in European countries, their use and results in the reimbursement of medicines according to the ISPOR European Congress, and also directions of implementation and pharmacoeconomics of formulary system in Ukraine.
We implemented the system of “lifelong education on pharmacoeconomics” for pharmacists during postgraduate courses. After passing the certification of pharmacists continue to use educational materials from the theory of pharmacoeconomics with a website www.uspor.org [11]. If they do not have access to the Internet, pharmacists may read publications in scientific journals i.e. "Pharmaceutical Journal", "Clinical Pharmacy", "Pharmacist-Practitioner", "Pharmacist" and study terminology, current activities of ISPOR. Thus pharmacists themselves explore their knowledge of the terminology of the ISPOR Book of Terms presented on the website. In June 2012, the 7th Scientific Conference "Pharmacoeconomics in Ukraine” was held, which was attended by over 100 practical pharmacists.
It should be noted that the lectures and seminars of pharmacoeconomics for 2000-2011 academic years were held for more than 11 000 postgraduate pharmacists in Lutsk, Lviv, Ivano-Frankivsk, Uzhhorod, Rivne, Ternopil, Khmelnytsky and Chernivtsi regions.
Summary
Reforming higher pharmaceutical education requirements under the Bologna Declaration should help prepare pharmacists to use modern disciplines, master new technologies and new knowledge by using the credit-module system. The introduction of lifelong education of pharmacists on “Pharmacoeconomics” using distance learning demonstrates the relevance and appropriateness of skills acquisition pharmacoeconomic analysis of the system of postgraduate training for pharmacists.
The modern pharmacist is free to navigate the scientific information, to be able to distinguish between materials of questionable value against reliable and useful data that serve as the basis to make management decisions in health care and with the help of an individual patient.
- Anderson C., Bates I., Beck D. et al. FIP and WHO move forward in developing pharmacy education. International Pharmacy Journal. 2008; 1(8): 3-5
- Rascati KL., Drummond M F., Annemans L., Davey PG. Education in pharmacoeconomics: an international multidisciplinary view. Pharmacoeconomics. 2004; 22: 139-147
- Rotger R. Trends, Perspectives and Pharmacy Education. International Pharmacy Journal. 2008; 1(8): 11-12
- Wiffen F. Evidence-based pharmacy. 2001; Radcliffe Medical Press. 182 p.
- Zalis’ka O. The theoretical basis of Pharmacoeconomics and practical use in Ukraine. Doctoral Dissertation. Lviv. 2004: - 367 p. Available from: www.disser.com.ua/content/348479.html
- Zalis’ka O. Pharmaceutical Encyclopedia of Ukraine. Available from: http://www.pharmencyclopedia.com.ua/article/1551/zaliska-olga-mikolaivna
- Zalis’ka O. Pharmacoeconomics / edited. BL Parnovsky. Textbook. – Lviv.- 2000. - Part.1.- 64 p., Part 2.- 71 p
- Zalis’ka O. Pharmacoeconomics / edited. BL Parnovsky.- Textbook. Lviv, 2007. 400 p
- Zalis’ka O. Pharmacoeconomics / edited. BL Parnovsky.- Textbook. Lviv, 2007. 376 p
- Mostovyy Y., Tomashkevych I., Konstatynovych-Chichirelo T. Pharmacoepidemiological and pharmacoeconomic research in medicine: Textbook. Vinnitsa, 2003; 79 p
- The curriculum of lifelong education on pharmacoeconomics for pharmacists of specialties "Organization and management of pharmacy", "General Pharmacy" between pre-cycle / [O. Zalis’ka, M.Slabyy, B.Parnovsky, I.Mudrak]. Lviv, 2009. 19 p
- MOH of Ukraine of 07.07.2009, № 484 "Amendments to the Regulations on examinations for certification courses, approved by the Ministry of Health of Ukraine of 18.05.94 no 73". Available from: http://www.moz.gov.ua/documents/
- Yakovleva L., Bezdetko N., Herasymova O. Pharmacoeconomics. Tutorial. Kharkiv, 2006. 119 p