How to optimize public spending on antihypertensive treatment in Poland - an example of rationalization analysis
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Tomasz Hermanowski |
Department of Pharmacoeconomics, Medical University of Warsaw, Warszawa, Poland |
|
Łukasz Borowiec |
Medical Department, Servier Polska, Warszawa, Poland |
|
Tomasz Faluta |
Medical Department, Servier Polska, Warszawa, Poland |
Background: Approximately 13% of global deaths are assigned to high blood pressure. ACE-Is and ARBs belong to most frequently prescribed classess of antihypertensive treatment. Recent meta-analyses have confirmed lack of evidence for predominance of ARBs over ACE-Is. Nevertheless, in Poland ARBs remain premium priced and better reimbursed compared to ACE-Is.
Objective: To assess economic impact of combining existing separate limit groups of the RAAS inhibitors into one group. Presented analysis is an example of rationalization analysis – a new type of analysis introduced by the reimbursement law 2011.
Methods: Reimbursement spending in one year horizon was assessed in two scenarios, assuming separate and common limit groups for ACE-Is and ARBs. List of products analysed and their unit prices are based on MoH listing of reimbursed drugs for 1 July 2013. Yearly volume of reimbursed packs was based on the most recent available data ie. NHF reports May 2012 to April 2013.
Results: Yearly savings from the public payer perspective is estimated at 155 mln PLN, a significant fraction (2.3%) of the actual spending on drug reimbursement. Average cost of reimbursement of a monthly therapy using ACE-Is and ARBs is estimated at 2.22 and 3.85 PLN respectively, as compared to 2.35 and 10.85 PLN prior to the change.
Conclusion: Combining ACE-Is and ARBs into a common limit group could ensure significant savings for the payer without compromising public health. Existing clinical evidence suggests that current practice of financial preference of ARBs over ACE-Is may lead to suboptimal allocation of the public resources.
Introduction
From the beginning of the year 2012, most of the provisions of the Act on Reimbursement of Medicines, Foodstuffs for Special Nutritional Purposes and Medical Devices (Reimbursement Act) came into effect in Poland [1]. Having considered its multidimensional influence on almost all participants of the Polish healthcare system, it constitutes one of the most significant reforms introduced in Poland over the past few years. Given its fundamental objectives, i.e. rationalization of the National Health Fund (NHF) expenditures on reimbursement and facilitation of access to drugs, including innovative drugs in particular, the Act appears to be parallel to the activities undertaken in other European countries [2-4].
The Reimbursement Act introduced the restriction on the NHF expenditures on drugs to 17% of the total resources directed to the financing of guaranteed services in the NHF financial plan. As a consequence, the financial policy became stricter. In particular, access to the drugs is regulated to a large extent by the mechanism of therapeutic reference pricing. Drugs which have the same international nonproprietary name (INN) or different INN but have a similar therapeutic effects and similar mechanism of action, could be classified into common limit group, based on the criteria of same reimbursed indications and similar efficacy. Thus, the Act grants the possibility to develop extensive limit groups, including above all the therapeutic indications specified in the Summary of Product Characteristics and related clinical efficacy and not only the active substance.
If justified, modifications within already existing limit groups are also acceptable. The organ entitled to implement such modifications is the Minister of Health (MoH). The fundamental-advisory role in this respect are played by the President and the Transparency Council of the Agency for Health Technology Assessment in Poland (AHTAPol). Based on the comparison of the health effects or additional health effects obtained, it may recommend introducing changes in the limit groups.
New regulation has also modified the manner of establishing base for the limit. The reference point in a given limit group does not constitute the price of the cheapest drug, as it was done earlier, but the medicine which is representative for the particular limit group (as quantitative market share amounting to 15% in this limit group). The list of reimbursed drugs (with the level of reimbursement, limit groups, patient’s contribution and the base of financing limitation) is currently published in the form of the MoH announcement every two months.
The Reimbursement Act [1] introduced a new type of analysis - rationalization analysis, required in case the budget impact analysis (BIA) for a health technology submitted for reimbursement indicates an increase in the payer’s reimbursement cost. The rationalization analysis should provide solutions, the inclusion of which in the reimbursement will result in a release of public funds at an amount which corresponds to at least the increase in the costs arising from the BIA.
RAAS inhibitors in hypertension treatment - are they equivalent?
In Poland, among the most frequently prescribed drugs are the ones for the treatment of cardiovascular related diseases, including antihypertensive drugs. Cardiovascular diseases (CVD) are the leading cause of mortality in Europe and worldwide with 48% of all deaths attributable. According to the statistics, an estimated 80 million people in Europe have greater than one in four, 10-year risk of a vascular event [5,6]. High BP is the main risk factor leading in 49% to ischemic heart disease and in 62% to stroke. Moreover, approximately 13% of global deaths are assigned to this manifestation [5,7,8]. Given the fact that an estimated 44% of Europeans over 35 years suffer from hypertension, the primary objective of current European hypertension guidelines, ie. blood pressure (BP) reduction aiming at cardiovascular mortality and morbidity decrease appears to be fully justified [9,10].
Proven effectiveness of nonpharmacologic interventions in lowering BP has its reflection in Polish and worldwide recommendations [9,11,12]. Irrespective of that, application of medication in many individuals is inevitable. The existing and widely used antihypertensive drugs incorporate renin angiotensin aldosterone system (RAAS) inhibitors, calcium channel blockers, beta-blockers and the diuretics [13,14]. They are licensed for initiation or maintenance of hypertension treatment and applied in monotherapy or in combination with other medicines [9]. Having regard to comorbidities and particularities of patients as well as specific properties, advantages and limitations assigned to the particular class of drugs, treatment should be individualized to achieve maximum therapeutic effect [9].
Having considered the role of the RAAS in regulation of homeostasis, arterial pressure, tissue perfusion and extracellular volume [15], drugs related to its blockade: angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) have pivotal role in the treatment of hypertension in routine medicinal practice [2,5]. They are considered to be clinically equivalents, although it is equivocal proclamation as they both block RAAS by different ways of operation. While ACE-Is prevent the enzyme ACE from converting angiotensin I into II, ARBs prevent the binding of angiotensin II to AT1 receptor [15,16]. Additional ACE-Is features of clinical implication are inhibiting degradation of the bradykinin resulting in vasodilatation and its potential beneficial role in cardiac protection, but also some adverse events not attributable to ARBs: dry cough (occurring in 5- 20 % of the patients) and angioedema (observed in 0.1% to 0.2% patients) [15,16].
A number of randomized controlled trials studying the ACE-Is and ARBs separately proved their high efficacy in reduction of mortality, myocardial infarction, stroke, heart failure and readmissions in patients suffering from heart failure [17], with stated left ventricular dysfunction [18-20], at high-risk and with vascular disease history [21-23] and at high-risk diabetes [24]. The direct and indirect comparisons of the two classes of drugs under the meta-analysis may settle their clinical equivalence.
Having regard to blood pressure reduction, meta-analysis of 2008 demonstrated that ACE-Is and ARBs are of clinical equivalence. This head to head comparison conducted on a group of adult patients with essential hypertension suggested that discussed therapies provide similar antihypertensive effect [25]. No significant differences in the frequency of selected end points, ie. death, cardiovascular events, major adverse events and quality of life were presented. No particular groups of patients of higher effectiveness, better tolerance or less frequency of adverse events occurrence were identified. However, it was stated that the use of ACE inhibitors is linked with more frequent occurrence of cough. Presumably following that, ARBs were associated with higher rates of persistence with initial therapy than ACE-Is [25].
Effectiveness of ACE–Is and ARBs as drugs used additionally in a standard therapy in stable ischemic heart disease with preserved ventricular function was compared in systematic reviews of 2009 and 2011 [26,27]. According to those publications, evidence on reduced mortality, myocardial infarctions and stroke were assigned to ACE-Is only. No additional effects were attributed to neither using ARBs nor by combining an ACE inhibitor and an ARBs [26,27].
The predominance of ACE-Is over ARBs was identified in the treatment of patients with diabetes in systematic review of 2008 [28]. Review included randomized controlled trials (RCT) of antihypertensive drugs among hypertensive or normotensive patients suffering from diabetes without nephropathy and RCT of ACE-Is or ARB in patients with diagnosed diabetic nephropathy. It was revealed that ACE-Is are the only drugs having positive renal effect in patients with diabetes without nephropathy as well as were associated with proven survival benefit in patients with diabetes and nephropathy [28].
Landmark conclusions were provided by meta-analysis conducted in 2012, the first attempt to assess the RAAS inhibitors influence on mortality in hypertension as basic indication. Comparison of RAAS inhibitors with another antihypertensive treatment or placebo in the group of 158,998 hypertensive patients allowed to conclude that RAAS inhibitors resulted in the decrease of all-cause mortality [29]. It should be emphasized however that gained health effects were constrained to the ACE-Is and were not observed for ARBs; stratified subgroup analysis showed significant 10% relative reduction in all-cause mortality associated with the usage of ACE-Is compared with no mortality reduction observed for ARBs [29].
The results of the meta-analysis of 2013, comparing ARBs and ACE-Is versus placebo in 108,212 patients without heart failure was consistent with the previous results. Unlike ARBs, ACE-Is significantly reduced not only all-cause deaths but also new onset of heart failure and diabetes mellitus [30]. Meta-analysis showed no advantage of ARBs over ACE-Is in reducing the risk of the composite outcome of CV death, MI and stroke. Taking the above results into account, one of the main conclusion is ARBs approval as a therapeutic substitute to reduce CV mortality and morbidity in patients for whom ACE-Is cannot be applied, eg. for patients experiencing ACE-inhibitor induced cough [30].
Reimbursement of RAAS inhibitors in Poland
Data reported by National Health Fund [44-46] confirm that RAAS inhibitors constitute an important class of products reimbursed in Poland, both in terms of the volume of yearly consumption (50.5 mln packs) and the level of public expenditure on reimbursement (430.5 mln PLN). Yearly number of reimbursed patient-months of therapy [1] is nearly 96 mln.
Under currently existing system, the RAAS blockers are available in Poland as separate limit groups with different methods of reimbursement. ACE-Is belonging to 44.0 [2] limit group are available for patients for lump sum up to the defined limit above which patient additional payment is required. ARBs belonging to 45.0 limit group are reimbursed at 30% copayment up to a defined refund limit. At the moment of analysis (July 2013), the 44.0 group consists of 138 products (EAN codes) containing either monotherapy or a fixed-dose combination of ACE-Is with a diuretic or a calcium-channel blocker. The 45.0 group consists of 194 products containing either monotherapy or fixed-dose combination with hydrochlorothiazide.
It should be emphasized that the fixed-dose combinations of ACE-Is are reimbursed only up to a limit calculated based on the amount of the ACE-I contained in the pack, and therefore generate additional savings for the payer resulting from reduced number of reimbursed packs of the diuretic or calcium-channel blocker. This is not the case for reimbursed fixed-dose combinations of ARBs (hydrochlorothiazide is not reimbursed from public funds).
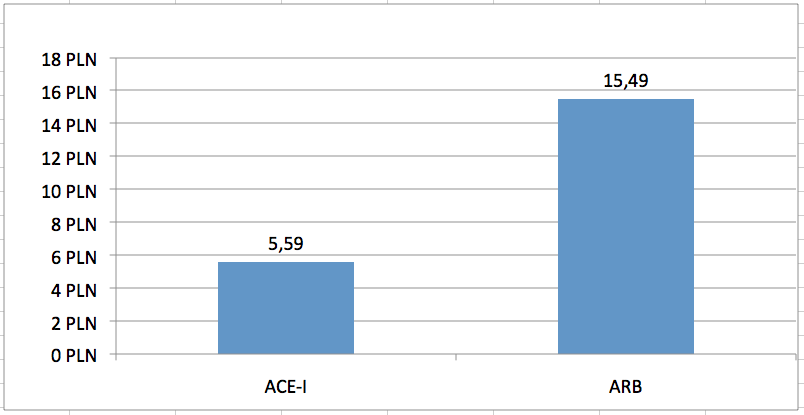
Consequences of separate limit groups are different upper limit funding used in relation to the discussed groups of drugs. Average limit of financing a monthly therapy [30 DDDs] from public funds is 2.8 times higher for ARBs vs ACE-Is (Fig 1). This results in significantly different level of public payer expenditure for both classes. Average cost of reimbursement from public funds of a monthly therapy [30 DDDs] is 4.6 times higher for ARBs vs ACE-Is (Fig 2). This translates into macro-scale – on a yearly basis (May 2012 to April 2013), the number of patient-months of therapy reimbursed from public funds amounted to 75.3 mln and 20.6 mln, respectively for ACE-Is and ARBs (95.6 mln patient-months of therapy with both classes). Public spending reported by National Health Fund was 190.8 mln PLN and 239.6 mln respectively for ACE-Is and ARBs, and over 430 mln PLN for products in both groups. This means that 56% of public expenditure for RAAS inhibitors was spent for reimbursement of ARBs despite only 22% share in the treatment of patients (Table 1).
Table 1. Number of packages of ACE-Is and ARBs reimbursed in Poland May 2012 to April 2013, along with the reimbursement spending reported by National Health Fund
|
Sources: authors’ calculations
Taking into consideration lack of clinical evidence confirming predominance of ARBs over ACE-Is and proven additional effects resulting from ACE-Is only, premium pricing of the ARBs is unjustified. One of the proposed solution to eliminate financial favoritism of ARBs could be combining the two limit groups, ACE-Is and ARBs into one limit group. As a consequence, one common reimbursement limit would be defined.
The objective of the analysis is to assess the direct financial consequences following implementation of the solution consisting in combining two existing limit groups representing the RAAS inhibitors, ie. ACE-Is and ARBs, belonging to the groups 44.0 and 45.0 respectively, into one limit group while retaining the current reimbursement schemes, ie. lump sum payment in relation to ACE-Is and reimbursement of 70% with reference to ARBs (proposed scenario). The resulting spending are to be confronted with expenditures under currently applicable conditions ie. coexistence of two separate limit groups (existing scenario).
Methods
The analysis was performed from the public payer perspective. The time horizon was one year. The only costs included in the analysis are those related to the NHF spending on reimbursement of drug prices, as the proposed solution does not affect any other related fields of the healthcare system.
For the purpose of the study, assumptions indicating a static market model were adopted in the analyzed time horizon, ie. the reimbursement schemes applicable to analysed products existing at the time of analysis would not be subject to modifications (lump sum payment for ACE-Is and reimbursement of 70% for ARBs will not change). The selling prices of analysed products were assumed constant in both scenarios and are based on the official reimbursement listing valid at the moment of analysis, ie. 1 July 2013 [31].
To estimate economic impact resulting from combining two limit groups into one common group, a new drug constituting a base for limitation for both drug categories will be determined. The limiting drug is designated consistent with the art. 15 para 4 of the Reimbursement Act [1]. According to the Act, the base for limitation in a given limit group constitutes the highest among the lowest wholesale price for DDD of medicine which covers at least 15% of monthly quantitative turnover achieved in this limit group, in a month which is 3 months prior to the publication of the MoH announcement. For the purpose of the analysis, drug serving as a base for limitation in the new limit group will be stated having regard to the value of drug reimbursement under the EAN codes as in March 2013. The value of reimbursement of March 2013 will be calculated as the difference between the value of drugs reimbursement of January-March 2013 and January-February 2013 [32,33]. The official price of the product serving as a basis for limitation in the new limit group will be adopted on a basis of the announcement of the Ministry of Health of 24 June 2013 on the list of reimbursed medicines, foodstuffs for special nutritional purposes and medical devices as of 1 July 2013 [31].
Taking into account the new drug serving as a base for limitation, annual reimbursement expenditures will be assessed. The annual consumption of drugs will was assumed based on the most recent data published in announcements of the Department of Medicines Policy of NHF. Number of boxes reimbursed over the period May 2012 to April 2013 is calculated based on cumulative reports for January-April 2013 [34], January-December 2012 [35] and January-April 2012 [36]. All analysed products pricing and unit reimbursement costs were determined using official sales prices defined in the reimbursement listing valid since 1 July 2013 [31], taking into account appropriate wholesale margin and retail margin considering the new limit base, as defined in the Reimbursement Act [1] in art 7 para 4.
All unit costs and total spending figures are expressed in the local currency (PLN); at the time of drafting the article (July 2013) the average official exchange rate of National Bank of Poland was 1 EUR=4.2756 PLN [37].
Results
In order to standardize different pack sizes and available doses of the analysed products, the number of packs reported by NHF was recalculated into numbers of “standard packs” containing 30 DDDs of the reimbursed molecule. This might be interpreted as the number of patient-months of therapy with the given product. The number of standard packages reimbursed on an annual basis was assessed to be 75.3 mln and 20.6 mln, respectively for ACE-Is and ARBs (Table 2). While maintaining the present method of reimbursement (ie. reimbursement in two separate groups), the yearly reimbursement level is estimated at 177.3 mln PLN and 223.9 mln respectively for ACE-Is and ARBs, which in total amounts to 401.2 mln PLN. Therefore the average payer cost of one ACE-I package is 2.35 PLN and 10.85 for and ARBs package - 4.6 times difference in unit costs (Fig 2).
Table 2. Comparison of the two scenarios (existing-separate limit groups for ACE-Is and ARBs against proposed solution - combining the two limit groups into common group) in terms of estimated NHF spending in annual perspective
|
||||||||||||||||||||||||||||||||
Sources: authors’ calculations
In the case of combining ACE-Is and ARBs in one limit group, the medicine which would meet 15% quantitive turnover calculated according to defined daily dose (DDD) for two groups of drugs would be Vivace 10 mg, 30 tabs. The official selling price of the medicine being a new basis for limit is PLN 16.09, with wholesale price PLN 17.06 and estimated retail price PLN 21.97. If ACE-Is and ARBs were combined in one limit group, the yearly reimbursement would amount to 167.0 mln PLN for ACE-Is and 79.4 mln PLN for ARBs, with the total reimbursement sum being 246.3 mln PLN. The unit reimbursement cost of ACE-Is and ARBs package is estimated at PLN 2.22 and 3.85, respectively.
The potential savings for the public payer in Poland resulting from the combining of ACE-Is and ARBs (group limits 44.0 and 45.0) in one group limit was assessed to be 155 mln PLN per year.
Limitations
The limitations identified in the present analysis are with regard to the adopted static market model in annual perspective. It concerns the aspects associated with proportional consumption of drugs, drug prices and the drugs indicated to be the basis for limitation in the studied limit groups.
The adopted assumption results from the lack of appropriate tool enabling to perform more precise simulation of modifications observed in the future. The announcement of the MoH is updated every two months. It is connected with high changeability of the market, frequent modifications of drugs prices and the limit base in therapeutic groups (eg. if the share of the inexpensive drugs rise on the market, the limit is decreasing) which cannot be precisely modelled.
Disscussion
Given the limited funds assigned to guaranteed services in the NHF financial plan, rational decisions not only cost-effective but also clinically-efficient should be the only ones acceptable. This paper has stressed several essentials of great necessity of change in Poland.
The existing algorithm of RAAS-drugs grouping results in significant premium pricing of ARBs over ACE-Is. Public payer actual spending per one standard package is 4.6 times higher for ARBs than ACE-Is. Taking into account the similar effectiveness of ARBs and ACE-Is in BP reduction, proven additional clinical effects - reduction in mortality resulting from application of ACE-Is [29] and additional health benefits attributable to the usage of ACE-Is in selected groups of patients [26-30], higher acquisition costs of the ARBs is unreasonable. Proposed solution in the form of combining ACE-Is and ARBs into one limit group, could serve for elimination of the ARBs premium pricing, and thus rationalize public reimbursement spending.
Among the advantages of the proposed solution are immediate release of funds, independent from influencing the supply and demand-side, including eg. intervening in clinical decisions regarding the administration of medicines for patients by the physicians. Estimated savings for the public payer as a consequence of passing postulated administrative decision were assessed to be 155 mln PLN annually. This might be a conservative estimate taking into account the recently observed trends in the RAAS inhibitors prescription patterns (decrease of ACE-Is and increase of ARBs), especially after implementation of the Reimbursement Law which changed ARBs reimbursement level from 50% to 70%.
Proposed solution appears to be consistent with the mechanism of classifying drugs into limit groups defined under Reimbursement Act. Despite different INN, ACE-Is and ARBs might be considered to be clinical substitutes; having regard to similar pharmacodynamics and the therapeutic indications. As an example in the NICE reccomendation they have the same position in therapeutic schemes [12,38]. Moreover, due to the analogous pharmacodynamics, dual blockade of the RAAS by combining these two groups of drugs in the patients suffering from hypertension is not recommended [12,38]. Irrespective of existing favorable effects, use of dual treatment is unsuccessful in mortality reduction. Additionally, more frequently accruing hyperkalaemia, hypotension, and renal failure in comparison to monotherapy questions rationale of the dual therapy [40].
The findings of the latest meta-analyses which revealed the predominance of ACE-Is are reflected in the latest, updated worldwide recommendations. These indicate that ACE-Is are preferred to ARBs as therapeutic option. According to the recommendations of the Heart Foundation of 2012 [40], in patients suffering from coronary heart diseases (CHD) and those at high risk of recurrent events, prescribing ARBs should be constrained for patients intolerant to ACE-Is. It is also recommended that ACE-Is should be the first-line antihypertensives in patients with pre-existing CVD, diabetes, diabetes with proteinuria and hypertension [41]. The recommendations of the Heart Foundation of 2011 suggest prescribing ACE-Is for people with all grades of systolic heart failure, asymptomatic systolic LV dysfunction and as prevention in high risk people with a history of MI or other cardiovascular disease. ARBs are considered to be an alternative for people unable to tolerate ACE-Is [41]. In reference to the patient with stroke, recommendations of 2010 stated that application of ACE inhibitor singly or in combination with a diuretic is the most effective way of BP lowering [42].
According to the reimbursement decisions issued for the analysed products, they are subject to reimbursement from public funds only in case of prescription in approved therapeutic indications, ie. based on Section 4.1 of the Summary of Product Characteristics (SmPC). All approved therapeutic indication for ARBs are applicable for ACE-Is too. Among therapeutic indications not approved for ARBs but approved for ACE-Is we can find: myocardial infarction, secondary prevention after myocardial infarction, left ventricular dysfunction after myocardial infarction, coronary artery disease, ischemic heart disease (with left ventricular dysfunction), renovascular hypertension and chronic kidney disease (Table 3).
Table 3. Comparison of indications for ACE-I and ARB reimbursed in Poland [43]
|
In relation to European recommendations, tendency to favour ACE-Is in clinical decisions also can be noted in special patient populations:
- According to the NICE recommendations of 2010 it is advised to use ACE-Is as drugs of first choice with beta blockers in heart failure due to left ventricular systolic dysfunction. Recommendations suggest using ARBs as an alternative to ACE-Is for patients presenting side effects after application of ACE-Is [38].
-Under guidelines of the European Society of Cardiology (ESC) and European Society of Hypertension of 2007, ACE-Is are preferred over ARBs in the treatment of hypertension and asymptomatic arteriosclerosis [9];
- Recommendations of the Polish Association of Hypertension of 2011 suggest usage of ARBs as an alternative when ACE-Is cannot be applied in the treatment of hypertension with the coexisting ischemic heart disease or heart failure [11];
- Recommendations of ESC as of 2006 stated that ARBs may be applied as an alternative in the stable ischemic heart disease when ACE-Is cannot be applied with additional indications to inhibition of RAAS system [43].
Many countries decided already to improve RAAS drugs prescribing efficiency to obtain positive results in savings. They could serve as good example for Polish authorities, as latest legislation changes make up a background for positive modifications.
Austria was one of the first countries which decided to limit the prescribing of more expensive ARBs to patients intolerant to ACE-Is. The decision was made in consequence of lack of relevant data demonstrating increased effectiveness of ARBs versus ACE-Is justifying request of manufacturers for premium pricing. As a result, utilization of ARBs in Austria in 2007 accounted for approximately 27% of all RAAS inhibitors and was significantly lower than in Sweden (43%) at the same time [2,4].
In Sweden, reassessment of the value of almost 2,000 drugs incorporated in the national reimbursement scheme in 2008 resulted in restrictions put on 26 substances including all ARBs and one ACE-Is [4,14]. The absence of documented health benefits to rationalize higher ARBs price if ACE-Is were well tolerated determined the decision to reimburse ARBs only for patients intolerant to ACE-Is or as a complement to ACE inhibitors and reimburse Monopril for patients with seriously decreased kidney function. Consequently, the number of patients treated with ARBs declined by 24% and increased for ACE-Is by 14%. Upward trend in expenditures for ARBs was stopped and accounted for 4% in 2008 compared to the year 2006 and 2007 when 13% and 9% increase was noted, respectively [4,14].
In Canada, economic analysis, comparing direct cost in two scenarios with and without policy restrictions on the use of ARBs demonstrated potential budgetary savings following restricted access to ARBs to be 77 mln dollars per year [44].
In Croatia, a variety of measures aiming at moderating ARBs prescribing were implemented. Restrictions on prescribing of ARBs in second-line therapy; to patients intolerant to ACE-Is and non-specific solutions, addressed to all drugs ie. academic detailing, monitoring of issued prescription, financial penalties belonged to the major, potentially most influential modifications. In consequence, reimbursed expenditures per defined daily dose (Exp/DDD) of ACE-Is and ARBs from 2001 to 2007 in Croatia decreased from Euro 0.34 to Euro 0.22, EXP/DDD of all ARBs, administered in single and combination therapies decreased from Euro 0.69 to Euro 0.21,EXP/DDD of all ACE-Is declined from 0.33 to 0.21 [3].
The issue which needs further investigation in Poland is the proportion of ARBs consumption in comparison to ACE-Is. Referring to the NHF data, ARBs utilization is currently assessed to be about 22% in Poland (Table 1). From the perspective of clinical studies coughing, the predominant factor in charge of switching therapies, occurred in approximately 10% of patients to whom ACE-Is were prescribed. However, about 5 % of them discontinued usage of these drugs [45, 46]. Reduced mortality due to the usage of ACE-Is implies that they should be used as the medicines of first choice in the treatment of hypertension. The additional health benefit assessed in the absolute values to be 3.8 per 1,000 patient-year would simply result in saving of many human lives at a low costs.
The proposed policy might appear to have a financially negative impact on patients’ spending. However, setting a common limit for both classes of RAAS inhibitors will eliminate the currently existing financial incentive promoting treatment with no added clinical benefit demonstrated. Thus it is expected that the structure of utilization will be adjusted accordingly (ie. increased use of ACE-Is), which could generate additional health gain for the patients. In order to secure current copayment levels for the relatively small group of patients intolerant to ACE-I treatment, one could consider to maintain a separate limit on ARBs in case of documented intolerance to ACE-Is, and a common limit otherwise. This approach would only slightly reduce the estimated savings presented in our analysis.
Conclusions
There is a strong need to improve RAAS inhibitors prescribing efficiency in Poland. Combining ACE-Is and ARBs into one common limit group could trigger an estimated 155 mln PLN annually, while maintaining at least the currently existing clinical effectiveness. The estimated savings constitute a significant part of the total public spending on drugs in Poland – 2.3% [47]. The proposed solution is consistent with the applicable regulations, Reimbursement Act in particular, and does not interfere with individual clinical decisions of the health professionals. However, in the face of quoted clinical data, further restrictions towards excessive ARBs prescription should be considered. The modifications implemented in drugs policy in the EU and non-EU countries could serve as a benchmark to rationalize usage and pricing of RAAS inhibitors.
[1] 30 DDDs of the reimbursed molecule
[2] 44.0 and 45.0 are references to the system of numbering the limit groups, used in the official Announcements of the Minister of Health
Acknowledgment
Łukasz Borowiec i Tomasz Faluta are employees of Servier, manufacturer of ACE-I perindopril
- 1. Ustawa z dnia 12 maja 2011 r. o refundacji leków, środków spożywczych specjalnego przeznaczenia żywieniowego oraz wyrobów medycznych. (Dz. U. z 2011 r. Nr 122, poz. 696, z 2012 r. poz. 95, poz. 742 z późn. zm)
- 2. Godman B., Bucsics A., Burkhardt T., Schmitzer M., Wettermark B., Wieninger P. Initiatives to enhance renin–angiotensin prescribing efficiency in Austria: impact and implications for other countries. Expert Rev. Pharmacoeconomics Outcomes Res 2010; 10 (2): 199–207
- 3. Voncina L., Strizrep T., Godman B. et al. Influence of demand-side measures to enhance renin–angiotensin prescribing efficiency in Europe: implications for the future. Expert Rev. Pharmacoeconomics Outcomes Res 2011; 11(4): 469–479
- 4. Wettermark B., Godman B., Neovius M. Initial effects of a reimbursement restriction to improve the cost-effectiveness of antihypertensive treatment, Health Policy 2010; 94: 221–229
- 5. World Health Organization. The World Health Statistics 2012. World Health Organization. 2012. p.35. Available from: http://apps.who.int/iris/; [Accessed: 10 March 2013]
- 6. Kaplan W., Laing R. Priority Medicines for Europe and the World Project. A public health approach to innovation. WHO, Geneva, Switzerland. 2004. Available from: http://whqlibdoc.who.int/hq/2004/WHO_EDM_PAR_2004.7.pdf; [Accessed 10 March 2013]
- 7. Kochanek KD., Xu JQ., Murphy SL., Miniño AM., Kung HC. Deaths: final data for 2009. National vital statistics reports. 2011; 60(3). Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf; [Accessed 10 March 2013]
- 8. Ezzati M., Hoorn SV., Rodgers A., Lopez AD., Mathers CD., Murray CJ. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet 2003; 362: 271–80
- 9. Mancia G., De Backer G., Dominiczak A. et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the EuropeanSociety of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28: 1462.1536
- 10. Cutler JA., Sorlie PD., Wolz M., Thom T., Fields LE., Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008; 52: 818–27
- 11. Widecka K., Grodzicki T., Narkiewicz K., et al. Zasady postępowania w nadciśnieniu tętniczym - 2011 rok. Wytyczne Polskiego Towarzystwa Nadciśnienia Tętniczego
- 12. NICE clinical guideline 127. Hypertension. Clinical management of primary hypertension in adults. 2011. Available from: http://www.nice.org.uk/nicemedia/live/13561/56008/56008.pdf; [Accessed: 12.03.2013 r.]
- 13. Olafiranye O., Zizi F., Brimah P., Jean-Louis G., Makaryus AN., McFarlane S., Ogedegbe G. Management of Hypertension among Patients with Coronary Heart Disease. Int J Hypertens. 2011; 2011: 653903. doi: 10.4061/2011/653903
- 14. Hedberg N., Jacob J. A review of medicines for lowering blood pressure - summary. Solna: Dental and Pharmaceutical Benefits Agenc. 2008. Available from: http://www.tlv.se/Upload/Genomgangen/review-blood-pressure.pdf; [Accessed April17 2009]
- 15. Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007; 13: 9.20
- 16. Israili ZH., Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992; 117: 234-42
- 17. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325: 293-302
- 18. Pfeffer MA., Braunwald E., Moyé LA., et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement trial. N Engl J Med. 1992; 327: 669-77
- 19. Jong P., Yusuf S., Rousseau .F., Ahn SA., Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003; 361: 1843-8
- 20. Flather MD, Yusuf S, Køber L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet. 2000; 355: 1575-81
- 22. Fox KM. EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003; 362: 782-8
- 23. Dagenais GR., Pogue J., Fox K., Simoons ML., Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet 2006; 368: 581-8
- 24. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000; 355: 253-9
- 26. Baker WL., Coleman CI., Kluger J., et al. Systematic Review: Comparative Effectiveness of Angiotensin - Converting Enzyme Inhibitors or Angiotensin II–Receptor Blockers for Ischemic Heart Disease. Ann Intern Med. 2009. 15; 151(12): 861-71
- 27. White CM., Greene L. Summary of AHRQ's comparative effectiveness review of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers added to standard medical therapy for treating stable ischemic heart disease. J Manag Care Pharm. 2011; 17(5 Suppl): S1-15
- 28. Strippoli G., Craig M., Schena F., et al. Role of blood pressure targets and specific antihypertensive agents used to prevent diabeteic nephropathy and delay its progression. J. Am. Soc. Nephrol. 2006; 17: S153–S155
- 29. van Vark LC., Bertrand M., Akkerhuis KM., Brugts JJ., Fox K., Mourad JJ., Boersma E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of rennin - angiotensin-aldosterone system inhibitors involving 158 998 patients. Eur Heart J. 2012; 33: 2088–2097
- 30. Savarese G., Costanzo P., Cleland JG. et al. A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013; 61(2): 131-142
- 31. Obwieszczenie Ministra Zdrowia z dnia 24 czerwca 2013 r. w sprawie wykazu refundowanych leków, środków spożywczych specjalnego przeznaczenia żywieniowego oraz wyrobów medycznych. Dziennik Urzedowy Ministra Zdrowia, Warszawa, dnia 24 czerwca 2013 r., Poz. 24
- 33. Komunikat DGL z dnia 28.05.2013 r. Wartość refundacji cen leków według kodów EAN (styczeń-luty 2013). Available from: http://www.nfz.gov.pl/new/index.php?katnr=0&dzialnr=2&artnr=5465; [Accessed: 29.07.2013]
- 34. Komunikat DGL z dnia 24.07.2013 r. Wartość refundacji cen leków według kodów EAN (styczeń-kwiecień 2013). Available from: http://www.nfz.gov.pl/new/index.php?katnr=0&dzialnr=2&artnr=5553; [Accessed: 29.07.2013]
- 35. Komunikat DGL z dnia 18.07.2013 r. Wartość refundacji cen leków według kodów EAN (styczeń-grudzień 2012). Available from: http://www.nfz.gov.pl/new/index.php?katnr=0&dzialnr=2&artnr=5537; [Accessed: 29.07.2013]
- 36. Komunikat DGL z dnia 16.07.2012 r. Wartość refundacji cen leków według kodów EAN (styczeń-kwiecień 2012). Available from: http://www.nfz.gov.pl/new/index.php?katnr=0&dzialnr=2&artnr=5012; [Accessed: 29.07.2013]
- 37. Available from: http://www.nbp.pl/kursy/archiwum/wagi.xls; [Accessed 07.08.2013]
- 38. NICE clinical guideline 127. Chronic heart failure Management of chronic heart failure in adults in primary and secondary care. Quick reference guide. 2010. Available from: http://www.nice.org.uk/nicemedia/live/13099/50517/50517.pdf; [Accessed 23 March 2013]
- 39. Makani H., Bangalore S., Desouza KA., Shah A., Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 2013; 346: f360
- 40. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand. Reducing risk in heart disease: an expert guide to clinical practice for secondary prevention of coronary heart disease. Melbourne: National Heart Foundation of Australia. 2012
- 41. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Quick reference guide. Diagnosis and management of chronic heart failure. Updated October 2011
- 42. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Quick reference guide. Diagnosis and management of chronic heart failure. Updated October 2011
- 43. Fox K., Garcia MA., Ardissino D., et al. Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG). Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006; 27(11): 1341-81
- 44. Guertin JR., Jackevicius CA., Cox JL., Humphries K., Pilote L., So DY., Tu JV., Wijeysundera H., Rinfret S. Canadian Cardiovascular Outcomes Research Team. The potential economic impact of restricted access to angiotensin-receptor blockers. CMAJ. 2011; 183(3): E180-6
- 45. Fletcher A., Palmer A., Bulpitt C. Coughing with angiotensin converting enzyme inhibitors; how much of a problem? J. Hypertens. 1994; 12: S43–S47
- 46. NHS. PCT prescribing report. November 2009. Prescribing of angiotensin-converting-enzyme (ACE) inhibitors and angiotensin-II receptor antagonists (AIIRAs) – prescribing guidance and discussion points
- 47. SPRAWOZDANIE Z DZIAŁALNOŚCI NARODOWEGO FUNDUSZU ZDROWIA ZA 2012 ROK. Warszawa, czerwiec 2012, s. 110
- 48. Available from: www.mp.pl; [Accessed: 07.08.2013]
21. Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting–enzyme inhibitor, ramipril, on cardiovascular events in highrisk patients. N Engl J Med. 2000; 342: 145-53
25. Matchar DB., McCrory C., Orlando LA., et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008; 148: 16-29
32. Komunikat DGL z dnia 26.06.2013 r. Wartość refundacji cen leków według kodów EAN (styczeń-marzec 2013). Available from: http://www.nfz.gov.pl/new/index.php?katnr=0&dzialnr=2&artnr=5505; [Accessed: 29.07.2013]






.png)






