Clinical effectiveness and cost-utility analysis of sunitinib for the treatment of pancreatic neuroendocrine tumors
-
Copyright
© 2012 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Jacek Walczak |
Arcana Institute, Krakow, Poland |
|
Magdalena Garbacka |
Arcana Institute, Krakow, Poland |
|
Joanna Jarosz |
Arcana Institute, Krakow, Poland |
|
Marzena Lipińska |
Arcana Institute, Krakow, Poland |
|
Justyna Zawieja |
Arcana Institute, Krakow, Poland |
|
Patrycja Prząda-Machno |
Pfizer Poland, Warsaw, Poland |
|
Jacek Kroc |
Pfizer Poland, Warsaw, Poland |
Background: The objective of this review is to assess the clinical effectiveness and cost–utility of sunitinib and best supportive care (BSC) versus placebo and best supportive care in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression.
Methods: Assessment of the clinical effectiveness of the interventions was conducted in accordance with the principles of systematic review, based on the Cochrane Collaboration guidelines (Cochrane Reviewer’s Handbook) and the guidelines of the Polish Agency for Health Technology Assessment (AOTM). The Markov model constructed in TreeAge Pro 2009 was used in the cost-utility analysis. The time horizon covered the period from the beginning of the treatment until the patient’s death (lifetime horizon). Quality adjusted years (QALY) were used as the measure of effectiveness and the results were presented as incremental cost-utility. CUA was conducted from the perspective of the public payer for health services (Polish National Health Fund, PNHF) and from the patient’s and PNHF’s perspective.
Results: As the result of the systematic search, one primary randomized clinical trial satisfying the inclusion criteria was found (Raymond 2011). The results of the present analysis clearly prove that sunitinib administered in a 37.5 mg dose is an effective and safe therapy in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression. The cost of gaining an additional QALY by replacing placebo+BSC with sunitinib+BSC is PLN 84,214 /PLN 84,296 (€20,441/€20,461) from PNHF/PNHF+patient perspective.
Conclusion: Sunitinib is a more costly and a more effective therapy than BSC.
Introduction
Pancreatic neuroendocrine tumors (pNETs) are uncommon tumors originating from highly specialized cells of the diffuse endocrine system. Those malignancies represent only 4% of all neuroendocrine tumors (NETs), of which over half are hormonally inactive tumors. PNETs belong to gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Up to 70% of all NETs are localized in the digestive system. The incidence of GEP-NETs has been estimated at 2.5 cases per 100 000, of which 10% are tumors of the pancreas, and 1/3 are clinically assessed as malignant [1]. Data published between 2008 and 2010 reports that pancreatic NETs are rarely occurring neoplasms of this organ constituting approximately 2% - 10%. The incidence of pets is estimated at 4 – 12 cases per million per year [2,3]. The peak incidence of pets is found in the fifth decade of life, with slight female predominance [3]. It is worth noting that most patients with pNETs (around 65%) have unresectable or metastatic disease at diagnosis [4].
It should be emphasized that rare diseases, such as the analyzed one, have been recognized as a priority area in public health in the European Union and given fundamental importance in European Union programs for health and scientific research. The assessment of a medical technology in reference to orphan drugs is a challenging issue in view of the frequent lack of comparative medicines and the small quantity of scientific reports due to the difficulties of conducting reliable studies on a small population.
Sunitinib malate (Sutent®), an oral multitargeted tyrosine-kinase inhibitor, is designed to inhibit: platelet-derived growth factor receptors (PDGFR-α and PDGFR-β), vascular endothelial growth factor receptors (VEGFR1, VEGFR2, VEGFR3), the stem cell factor receptor (KIT), fms-like tyrosine kinase 3 (FLT3), colony-stimulating factor-1 receptor (CSF-1R) and glial cell line derived neurotrophic factor receptor (RET). Thus, the analyzed drug influences cell growth, angiogenesis and tumor proliferation with metastases [5].
In the European Union (EU), sunitinib is indicated for the treatment of unresectable and/or metastatic malignant gastrointestinal stromal tumor (GIST) after failure of imatinib mesylate treatment due to resistance or intolerance, for the treatment of advanced/metastatic renal cell carcinoma (MRCC), and for the treatment of unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression. Sunitinib received marketing authorization in the United States on 26 January 2006 and in Europe on 19 July 2006 [5].
The therapy with multitargeted tyrosine-kinase inhibitor – sunitinib in the treatment of pNETs has already been recommended and reimbursed in such European countries as Great Britain, Switzerland, the Netherlands, France, and Finland.
The objective of this review is to comprehensively present clinical effectiveness and cost-utility (CUA) of sunitinib given as a support of best supportive care (SUN+BSC) in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression. The survey was conducted in accordance with the Cochrane Collaboration guidelines [6] and the Polish Agency for Health Technology Assessment (AOTM) recommendations [7]. The systematic review and CUA were conducted on the basis of a published high reliability randomized controlled trial (RCT) conducted in a double-blind manner.
Decision problem (PICOS)
The decision problem was formulated in accordance with the PICOS pattern (population, intervention, comparator, outcomes, study design):
Population: adults with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression;
Intervention: sunitinib administered orally at a dose of 37.5 mg per day (continuous regimen) in combination with best supportive care (SUN+BSC);
Comparator: placebo and best supportive care (PL+BSC);
Outcome: progression free survival (PFS), overall survival (OS), objective tumor response (complete response, partial response, stable response, progressive disease), objective response rate (ORR), quality of life, death, adverse events, quality adjusted life years (QALY);
Study design: head-to-head RCT trials conducted in parallel groups.
Clinical effectiveness analysis of sunitinib for the treatment of pNETs
Search strategy
The search strategy was designed by two independent authors. Terminology from scientific papers as well as from Medline Thesaurus (Mesh) was included. Boolean Operator (OR) was used to combine the search sets. Trials were identified by searching electronic databases such as: Medline via PubMed, Cochrane Library, EmBase, and CRD. To find additional primary studies that comply with the inclusion criteria, references of identified secondary literature were searched. Clinical Trial Registry - ClinicalTrials.gov was also screened. Terms used in the search strategy included, among others, phrases such as “sutent”, “sunitinib”, “sunitinib malate” and adequate synonyms. The search was conducted in March 2012.
At the stage of designing the search strategy, no restrictions regarding disease classification, alternative intervention and evaluated outcomes were adopted due to the possibility of lowering the sensitivity of the searching process applied. Also, no limitations were applied regarding publication type, which allowed identification of secondary and observational studies covering additional information in respect of practical efficacy and safety over a long period of time.
A two-step eligibility and selection process was used. The selection of relevant abstracts and full-text articles was prepared independently by two reviewers. Firstly, the reviewers independently screened all titles and abstracts to determine whether an article met the general inclusion criteria. Secondly, two reviewers independently assessed the full-text studies using predefined inclusion and exclusion criteria. The reference lists of identified articles were then examined for additional publications. Only published trials were included into the review. Data from abstracts or conference posters were accepted for inclusion into the analysis if those provided additional information to the full text published version.
Both authors independently extracted methodological data and outcomes; disagreements were resolved by discussion.
Selection criteria
The systematic review was performed in accordance with Evidence Based Medicine, contributions of the Cochrane Collaboration (Cochrane Reviewer’s Handbook) [6] and the guidelines of the Polish Agency for Health Technology Assessment [7]. The clinical question was formulated in accordance with the PICOS scheme (population, intervention, comparator, outcomes, study design). The primary endpoint analyzed in the included clinical trial was progression free survival defined as the time from randomization to the first evidence of progression or death due to any cause. Secondary efficacy endpoints included overall survival, objective response rate, time to tumor response, duration of response, safety, and patient-reported outcomes. Tumor response was assessed by investigators with the use of RECIST. Confirmed responses were those that persisted on repeat imaging 4 weeks or more after initial documentation. Safety analysis involved: discontinuation of the study, total adverse events, serious adverse events, and other adverse events. Safety assessments included documentation of adverse events with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. The self-administered European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30, version 3.0) was used to measure patient-reported outcomes.
Quality assessment
Quality assessment criteria included number of sites, randomization, presence of information concerning allocation concealment, blinding, and intention-to-treat (ITT) analysis. The validity of the clinical trial meeting the inclusion criteria for the analysis was determined with the use of the Jadad scale [8].
Statistical analysis
In the statistical analysis of dichotomous parameters, the Odds Ratio (OR) obtained in the compared groups was calculated. The Relative Risk (RR), the ratio of risk in the intervention group to the ratio of risk in the control group, was used as an effect measure. For variables of the "time to" (time to event) type, the hazard ratio (HR) was specified. In addition, as a measure of efficacy, the number needed to treat (NNT) was also calculated for the outcomes with significantly different overall effects. Mean Differences (MD) were calculated for continuous variables. All treatment effects were calculated within a 95% Confidence Interval (95% CI).
In the analysis of probability of rare events (in the case where at least in one of the groups there were events with a rate of occurrence of 0 or close to 0), calculations were performed using the Peto or Mantel-Haenszel methods.
All calculations were performed using the StatsDirect® 2.6.8 statistical package. A two-sided P-value of < 0.05 was considered significant.
Results
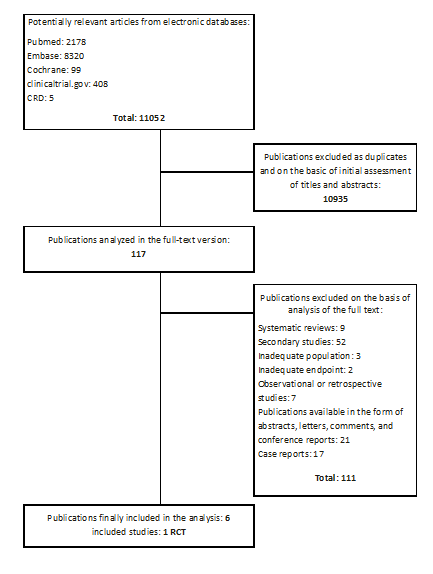
11 052 publications were identified (Pubmed: 2178, Embase: 8320, Cochrane: 99, CRD: 42, clinicaltrials.gov: 408, other: 5) of which 117 publications were analyzed in full-text version. As a result of a systematic search, one primary randomized clinical trial (phase III trial) satisfying the inclusion criteria was found (Raymond 2011) [4, 9, 10]. In addition, three conference reports [11, 12, 13], which contain updated results of Raymond 2011 study, were found and included into the analysis. Search results are demonstrated in Figure 1.
Date of the systematic search: 02 – 05.03.2012
The study Raymond 2011 included in the analysis was a multicentre, double-blind, randomized controlled trial (subtype II A). The Jadad scale [8] was used in the process of assessing the reliability of studies. The reliability of Raymond 2011 study is high and corresponds to 5 out of 5 points on the Jadad scale. The detailed characteristic of the included study are presented in Table 1.
Table 1. Characteristics of studies included in the analysis
| Study | Type of study | Number of centers | Inclusion criteria | Intervention | Endpoints | Jadad score |
| Raymond 2011 Sponsor:Pfizer | RCT, double blind | 42 | (1) histological or cytological proven diagnosis of well-differentiated pancreatic islet tumor (according to WHO 2000 classification) locally-advanced or metastatic disease; 2) disease not amenable to surgery; 3) documented disease progression within the previous 12 months as assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) with disease progression; 4) one or more measurable target lesions; 5) an Eastern Cooperative Oncology Group performance status of 0 or 1; 6) adequate organ function. |
SUN+BSC*: once-daily oral sunitinib at dose of 37.5 mg per day + best supportive care [N = 86]; PL+BSC*: matching placebo (1 placebo capsule identical to sunitinib per day) plus best supportive care [N = 85]; Treatmentcontinueduntil death, progression of disease, unacceptable toxicity. Patients with disease progression while receiving placebo could enter an open-label sunitinib extension protocol (NCT00443534 or NCT00428220). |
progression free survival, overall survival, objective tumor response, safety (deaths, discontinuations from the study, total adverse events, serious adverse events and other adverse events), quality of life. | 5 |
*Before the trial, during the trial, or both patients could receive somatostatin analogs at the investigator’s discretion
Patients with well-differentiated pancreatic neuroendocrine tumors were randomized into two groups receiving sunitinib (86 patients) or placebo (85 patients). All patients received best supportive care. Concurrent treatment with somatostatin analogs was permitted. Dose interruption and/or dose modification were permitted for toxicity. Treatment continued until death, progression of disease, or unacceptable toxicity. Patients with disease progression, while receiving placebo, could enter an open-label sunitinib extension protocol (NCT00443534 or NCT00428220).
Baseline characteristics were similar between the two study groups. Approximately 90% of patients in each treatment group had previously received surgery for pNETs. 66% (57/86) of sunitinib patients and 61% (72/85) of placebo patients had received previous systemic chemotherapy. The median duration of treatment in the phase III trial was 4.6 and 3.7 months in the sunitinib and placebo groups. Efficacy assessments were performed in the intent-to-treat population.
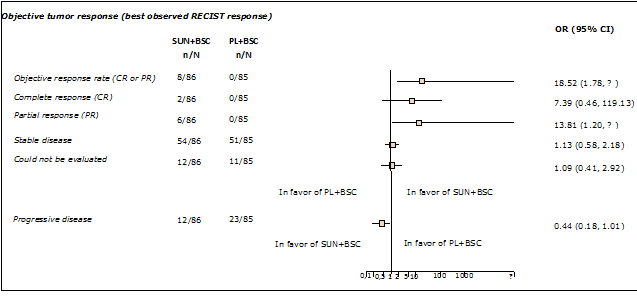
It was shown that the use of sunitinib plus BSC results in statistically significant higher clinical efficacy in respect of progression free survival (PFS), overall survival (OS) and objective response rate in comparison with the control group (placebo plus BSC). The median PFS (HR = 0.42; 95% CI, 0.26; 0.65) was over two fold greater in sunitinib treated patients (11.4 months) than in the placebo group (5.5 months). At the data cut-off point, 9 deaths (10%) were reported in the sunitinib group compared with 21 deaths (25%) in the placebo group (HR = 0.40; 95% CI, 0.18 to 0.86). Detailed data on the analyzed endpoints is shown in Table 2. The objective response rate was 9.3% in the sunitinib group versus 0% in the placebo group (Figure 2). Among the eight patients who achieved a tumor response (as assessed by RECIST) with sunitinib, two had a complete response and the remainder had partial responses; only one responder developed progressive disease before the trial was terminated. The time to tumor response ranged from 0.8 to 11.1 months (median 3.1 months) and responses lasted from 0.9 to > 15.0 months. The remaining sunitinib and placebo recipients had stable disease (63% vs 60%), progressive disease (14% vs 27%), or could not be evaluated (14% vs 13%) (Figure 2).
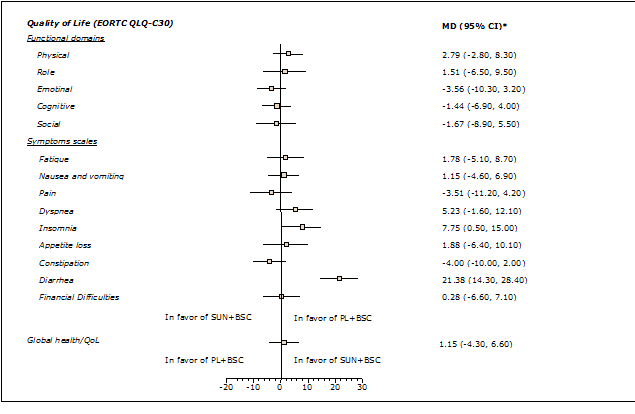
Health-related quality of life was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life questionnaire but the value of the results was limited by low patient numbers. There was no indication that treatment with sunitinib produced a significant deterioration in quality of life (Figure 3).
Sunitinib is a safe and well-tolerated therapy (Table 3). In the course of the analysis, statistically significant differences in favor of sunitinib plus BSC were indicated in the case of total death, discontinuation from the study due to progression or relapse and total serious adverse events. The most frequent adverse events in the sunitinib group were diarrhea, nausea, vomiting, asthenia and fatigue. In most cases, the analyzed adverse events were of low severity grade.
The Raymond 2011 study was closed prematurely, after the independent data and safety monitoring committee observed more serious events and death in the placebo group as well as a difference in PFS favoring sunitinib. A significant number of patients in the placebo arm crossed over to active treatment at progression or at early termination of the trial.
Crossover is common and unavoidable for ethical reasons, but leads to an underestimation of true clinical gain in OS with standard statistical analyses (intention-to-treat). Adjusting for crossover bias with the Rank Preserving Structural Failure Time (RPSFT) model amplified this effect: HR = 0.18 (95% CI: 0.06; 0.68); that is an 82% reduction in the risk of death with sunitinib compared placebo (Table 2).
A limitation of applying the RPSFT method to this study includes the relatively small size, however, findings provide a clear direction for the effect of crossover and the RPSFT result may provide a possible upper bound on the true effect size.
Updated OS and an estimate of the effect of sunitinib on OS by adjusting for treatment crossover was described in the poster Valle 2011. As of June 2010, median OS had been reached with 34 deaths in the sunitinib group and 39 deaths in the placebo group. Median follow-up time was 26.0 months. Updated ITT analysis of OS demonstrated a 6.1 month improvement in median OS in the sunitinib arm when compared with the placebo arm (HR = 0.737, 95% CI: 0.465; 1.168).
However, due to crossover, it could be underestimated and it did not reach statistical significance. When data were analyzed using the RPSFT model, OS reached statistical significance: HR = 0.499 (95% CI: 0.351; 0.947), that is a 50% reduction in the risk of death with sunitinib (Table 2).
Table 2. Efficacy results for progression free survival (PFS) and overall survival (OS)
| Outcome | Number of patients in SUN+BSC group | Number of patients in PL+BSC group | Number with events | Median, months (95 % CI) | Hazard ratio (95 % CI), p-value |
|
| Data cut-off point: 15 April 2009* | ||||||
| PFS | 86 | 85 | SUN+BSC: 30 PL+BSC: 51 |
SUN+BSC: 11.4 (7.4; 19.8) PL+BSC: 5.5 (3.6; 7.4) |
0.42 (0.26; 0.65), p = 0.000118 |
|
| OS | 86 | 85 | SUN+BSC: 9 PL+BSC: 21 |
Not reached | 0.40 (0.18 0.86) p = 0.02 |
|
| OS (model RPSFT***) |
- | - | - | - | 0.18 (0.06; 0.68) | |
| Data cut-off point: June 2010** | ||||||
| OS | 86 | 85 | SUN+BSC: 34 PL+BSC: 39 |
SUN+BSC: 30.5 (20.6; NA) PL+BSC: 24.4 (16.3; NA) |
0.737 (0.465; 1.168)p = 0.1926 | |
| OS (model RPSFT***) |
- | - | - | - | 0.499 (0.351; 0.947) p = 0.0035 |
|
*Raymond 2011 and Ishak 2011 [9,10]
**Valle 2011 [11]
***Model RPSFT (rank-preserving structural failure time) is a non-parametric model that produces a randomization-based effect estimator assuming that treatment with the investigational drug extends survival time uniformly for all patients. Assuming this model is correct, survival times can be calculated for all patients had they received placebo. For patients who crossed over, the time on treatment after crossover is adjusted to reflect what would have happened if they had stayed on placebo. Due to randomization, the distribution of the calculated survival times should be the same in both groups. The model has been recognized by health technology assessment bodies (e.g., the National Institute of Clinical Excellence in the UK, and Tandvårds-och läkemedelsförmånsverket in Sweden).
Cost-utility analysis of sunitinib for the treatment of pNETs
Analytical technique
In order to evaluate the profitability of well-differentiated pancreatic neuroendocrine tumors treatment using sunitinib (Sutent®) in combination with BSC in comparison to standard therapies reimbursed in Poland, a cost-utility analysis (CUA) was performed using the Markov decision model constructed in TreeAge Pro 2009. As the measure of effectiveness, QALY was used and the result was presented as incremental cost-utility ratio (ICUR). ICUR expresses the cost of gaining one additional unit of QALY in case of replacing BSC with sunitinib plus BSC. Additionally, a one-way sensitive analysis was performed to estimate the influence of fundamental, uncertain parameters (connected with costs, effects, methods or assumptions) on the results and conclusions. Furthermore, the best and the worst case scenarios were considered as multivariate analyses.
Table 3. Safety results (deaths, discontinuations, serious adverse events, common adverse events)
| Outcome | Intervention | N | n (%) | OR (95% CI) | NNT (95% CI) |
| Total deaths* | SUN+BSC | 86 | 9 (10.5) | 0.36 (0.13; 0.89) | 8 (4; 35) |
| PL+BSC | 85 | 21 (24.7) | |||
| Discontinued from the study due to adverse event | SUN+BSC | 86 | 15 (17.44*) | 2.35 (0.84; 7.20) | – |
| PL+BSC | 85 | 7 (8.24*) | |||
| Discontinuation from the study due to progression or relapse | SUN+BSC | 86 | 19 (22.0) | 0.23 (0.11; 0.47) | 4 (3; 6) |
| PL+BSC | 85 | 47 (55.0) | |||
| Discontinuation from the study due to death | SUN+BSC | 86 | 1 (1.16*) | 0.32 (0.01; 4.12) | – |
| PL+BSC | 85 | 3 (3.53*) | |||
| Total serious adverse events | SUN+BSC | 83 | 22 (26.5) | 0.51 (0.25; 1.03) | – |
| PL+BSC | 82 | 34 (41.5) | |||
| Diarrhea** | SUN+BSC | 83 | 49 (59) | 2.25 (1.15; 4.41) | 5 (3; 22) |
| PL+BSC | 82 | 32 (39) | |||
| Nausea** | SUN+BSC | 83 | 37 (45) | 1.94 (0.97; 3.90) | – |
| PL+BSC | 82 | 24 (29) | |||
| Asthenia** | SUN+BSC | 83 | 28 (34) | 1.39 (0.68; 2.87) | – |
| PL+BSC | 82 | 22 (27) | |||
| Vomiting** | SUN+BSC | 83 | 28 (34) | 1.16 (0.57; 2.36) | – |
| PL+BSC | 82 | 25 (30) | |||
| Fatigue** | SUN+BSC | 83 | 27 (32) | 1.31 (0.64; 2.72) | – |
| PL+BSC | 82 | 22 (27) | |||
| Hair-color changes** | SUN+BSC | 83 | 24 (29) | 32.95 (5.01; 1371.21) | 4 (3; 6) |
| PL+BSC | 82 | 1 (1) | |||
| Neutropenia** | SUN+BSC | 83 | 24 (29) | 10.71 (3.00; 57.44) | 4 (3; 7) |
| PL+BSC | 82 | 3 (4) | |||
| Abdominal pain** | SUN+BSC | 83 | 23 (28) | 0.83 (0.40; 1.70) | – |
| PL+BSC | 82 | 26 (32) | |||
| Hypertension** | SUN+BSC | 83 | 22 (26) | 7.03 (2.20; 29.24) | 5 (4; 9) |
| PL+BSC | 82 | 4 (5) | |||
| Palmar-plantar erythrodysesthesia** | SUN+BSC | 83 | 19 (23) | 11.88 (2.67; 107.60) | 5 (4; 9) |
| PL+BSC | 82 | 2 (2) | |||
| Anorexia** | SUN+BSC | 83 | 18 (22) | 1.06 (0.47; 2.40) | – |
| PL+BSC | 82 | 17 (21) | |||
| Stomatitis** | SUN+BSC | 83 | 18 (22) | 11.08 (2.48; 100.74) | 6 (4; 10) |
| PL+BSC | 82 | 2 (2) | |||
| Dysgeusia** | SUN+BSC | 83 | 17 (20) | 8.82 (1.92; 81.35) | 7 (4; 15) |
| PL+BSC | 82 | 4 (5) | |||
| Epistaxis** | SUN+BSC | 83 | 17 (20) | 8.82 (1.92; 81.35) | 7 (4; 15) |
| PL+BSC | 82 | 4 (5) | |||
| Headache** | SUN+BSC | 83 | 15 (18) | 1.42 (0.56; 3.68) | – |
| PL+BSC | 82 | 11 (10) | |||
| Insomnia** | SUN+BSC | 83 | 15 (18) | 1.59 (0.61; 4.23) | – |
| PL+BSC | 82 | 10 (12) | |||
| Rash** | SUN+BSC | 83 | 15 (18) | 4.30 (1.28; 18.51) | 8 (5; 27) |
| PL+BSC | 82 | 4 (5) | |||
| Thrombocytopenia** | SUN+BSC | 83 | 14 (17) | 3.96 (1.16; 17.16) | 9 (5; 38) |
| PL+BSC | 82 | 4 (5) | |||
| Mucosal inflammation** | SUN+BSC | 83 | 13 (16) | 2.35 (0.78; 7.94) | – |
| PL+BSC | 82 | 6 (7) | |||
| Weight loss** | SUN+BSC | 83 | 13 (16) | 1.51 (0.55; 4.25) | – |
| PL+BSC | 82 | 9 (11) | |||
| Constipation** | SUN+BSC | 83 | 12 (14) | 0.70 (0.28; 1.71) | – |
| PL+BSC | 82 | 16 (20) | |||
| Back pain** | SUN+BSC | 83 | 10 (12) | 0.67 (0.25; 1.74) | – |
| PL+BSC | 82 | 14 (17) |
*At the data cut-off point: April 15, 2009;
**Adverse events were defined on the basis of National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Events listed are those of any grade that occurred in more than 15% of patients in either group
Perspective
CUA analysis was conducted from the perspective of the public payer for health services (Polish National Health Fund, PNHF) and from the patient and PNHF perspective.
Time horizon
The time horizon covered the period from the beginning of treatment until the patient’s death (lifetime horizon).
Model structure
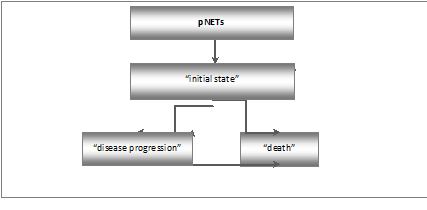
The model structure was based on the data from the clinical trial Raymond 2011 [9], analysis of the course of the disease, and medical expert opinion.
In the Markov decision model, the following states, which are important from economic or clinical point of view, were taken into consideration: “initial state”, “disease progression” and “death”. All patients entered the model in the “initial state” and were treated with either sunitinib plus BSC or placebo plus BCS. In the “initial state”, after the end of the cycle, a transition to the following states: “initial state”, “disease progression” and “death” is possible. The “disease progression” state can be followed after the end of the cycle by the “disease progression” or “death” state. The “Death” is the terminal (absorbing) state.
The length of the model cycle, corresponding to the frequency of health state changes in patients, is four weeks. A discount rate of 5% for costs and 3.5% for benefits was used.
Assumption and model parameters
Clinical data were taken from the main phase III clinical trial (Raymond 2011 [9]). Data on progression-free survival and overall survival were extrapolated using a Weibull method and included the use of RPSFT method to allow for crossover between the arms of the clinical trial. The base case included events occurring in the extension period of the clinical study, after un-blinding had occurred. This revealed an improved OS benefit (HR=0.18; 95% CI: 0.06–0.68) [10].
Patients in any state of the model (with the exception of the "death" state, which is an absorbing state) are exposed to the risk of progression, which increases as the disease progresses.
Utilities for the health states before (“initial state”) and after progression were based on a conversion of the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire responses from the clinical study Raymond 2011 into utility values. The “initial state” utility value was 0.73 and the progression utility value was 0.596 [9, 14].
Table 4 shows clinical parameters (effectiveness and utilities) used in the model.
Table 4. Summary of model parameters - effectiveness and utilities
| Parameters | SUN+BSC | PL+BSC |
| The shape parameter for OS | 1.63 | - |
| The scale parameter for OS | 40.04 | - |
| The shape parameter for PFS | 0.79 | 1.16 |
| The scale parameter for PFS | 19.89 | 6.31 |
| Utility “initial state” | 0.730 | 0.730 |
| Utility “disease progression” | 0.596 | 0.596 |
| P_death (1 cycle)* | 0.000000 | 0.000000 |
| P_ death (2 cycle)* | 0.002440 | 0.013481 |
| P_ death (3 cycle)* | 0.012963 | 0.069753 |
| P_ death (4 cycle)* | 0.028187 | 0.151328 |
| P_ death (5 cycle)* | 0.046766 | 0.244331 |
| P_ death (6 cycle)* | 0.067822 | 0.339491 |
| P_ progression (1 cycle)* | 0.000000 | 0.000000 |
| P_ progression (2 cycle)* | 0.087465 | 0.097843 |
| P_ progression (3 cycle)* | 0.137352 | 0.162067 |
| P_ progression (4 cycle)* | 0.172810 | 0.193011 |
| P_ progression (5 cycle)* | 0.198695 | 0.200967 |
| P_ progression (6 cycle)* | 0.217514 | 0.194445 |
* data taken from the model of cost-utility analysis
Following that, direct medical costs were included: sunitinib, the administration of the drug, diagnostic and monitoring, somatostatin analogs, BSC, severe adverse events (AEs) and palliative care. Only the cost of grade 3 and 4 AEs were considered. Prices were evaluated on the basis of Polish National Health Fund regulations applicable in 2012.
Median duration of drug use, which takes into account discontinuation due to an AE, disease progression and death, was used to estimate the cost of sunitinib and somatostatin analogs. The assumption that patients continue sunitinib treatment until the next disease progression was tested in a sensitivity analysis.
In the analysis, compliance at 91.3% in the group receiving sunitinib was included (estimated as the proportion of administered doses relative to the number of planned doses at 37.5 mg daily) [9].
Costs of terminal care (hospice at home within the last week of life) are associated with the “death” state.
Cost parameters used in the model are shown in Table 5.
Table 5. Summary of model parameters - costs
| Parameters | PNHF [PLN] | PNHF+patient [PLN] | ||
| SUN+BSC | PL+BSC | SUN+BSC | PL+BSC | |
| Cost of sunitinib /per cycle | 17,302.80 | - | 17,302.80 | - |
| Compliance | 0.913 | - | 0.913 | - |
| Cost of administration of the sunitinib | 104.00 | - | 104.00 | |
| Cost of diagnostic and monitoring: | ||||
| Computerized Tomography (CT scan) |
389.70 (every 2 months for the first six months, and then every 3 months until progression) |
- | 389.70 (every 2 months for the first six months, and then every 3 months until progression) |
- |
| Specialist advise (1st type) | 34.90 (every 4 weeks, or every 3 months in patients responding to treatment) |
- | 34.90 (every 4 weeks, or every 3 months in patients responding to treatment) |
- |
| Comprehensive advice (1st type) | 59.82 (in the first cycle) |
- | 59.82 (in the first cycle) |
- |
| Cost of monitoring | 34.90 (once every three months after the end of the program) |
34.90 (once every three months) |
34.90 (once every three months after the end of the program) |
34.90 (once every three months) |
| Cost of somatostatin analogues | 6,166.09 | 5,424.89 | 6,166.09 | 5,424.89 |
| Cost of BSC | 43.38 | 49.86 | ||
| Cost of severe adverse events | 393.85 | 18.03 | 394.78 | 18.15 |
| Cost of palliative care* | 1,501.64 | 1,501.64 | ||
* the cost of palliative care will be added during the last week of life
Results
Cost-utility analysis
Results of a cost-utility analysis of sunitinib plus BSC in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression were presented in Table 6.
Table 6. The results of the cost-utility analysis for a life time horizon
| Parameters | SUN+BSC | PL+BSC |
| PNHF perspective | ||
| Total costs[PLN] | 89,688.63 | 7,095.85 |
| Incremental cost [PLN] | 82,592.78 | |
| Total health effects [QALY] | 1.44 | 0.46 |
| Incremental health effects [QALY] | 0.98 | |
| ICUR [PLN/QALY] | 84,213.78 | |
| PNHF+patient perspective | ||
| Total costs[PLN] | 89,821.83 | 7,148.58 |
| Incremental cost [PLN] | 82,673.25 | |
| Total health effects [QALY] | 1.44 | 0.46 |
| Incremental health effects [QALY] | 0.98 | |
| ICUR [PLN/QALY] | 84,295.83 | |
The incremental cost-utility ratio (ICUR) for the comparison of sunitinib+BSC with BSC was determined from the following formula:
The cost of gaining an additional QALY by replacing placebo+BSC with sunitinib+BSC is PLN 84,214 /PLN 84,296 (€20,441/€20,461) from PNHF/PNHF+patient perspective. The cost-utility analysis proved that SUN+BSC is more expensive but more effective than BSC alone in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression.
The results obtained are below the acceptability threshold in Poland (which is about PLN 99,543 (€24,162)). The 2011 weighted average exchange rate of Polish National Bank was €1 = PLN 4.1198.
Sensitivity analysis
In order to investigate the influence of key parameter changes and the settings of the model on the results of the cost-utility analysis, one-way and multi-way sensitivity analyses were performed. A sensitivity analysis for the comparison SUN+BSC vs BSC showed robustness of the results (confirmed that SUN+BSC remains more expensive, but still more effective than BSC). The results were the most sensitive to:
- sunitinib was continued until disease progression (ICUR PLN 254,372.88 per QALY/ PLN 254,454.93 per QALY from PNHF/PNHF+patient perspective);
- minimal median duration of sunitinib use (ICUR PLN 16,239.56 per QALY/ PLN 16,321.61 per QALY from PNHF/PNHF+patient perspective);
- maximal median duration of sunitinib use (ICUR PLN 239,474.98 per QALY/ PLN 239,557.03 per QALY from PNHF/PNHF+patient perspective).
Discussion
The aim of this publication was to evaluate the clinical and cost effectiveness of sunitinib and best supportive care in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression on the basis of a systematic literature review.
When assessing the restrictions of this systematic review and CUA features, PICOS predefined inclusion criteria and the quality of input data available, as well as the scope of the analysis in respect of an explicit decision problem, should be considered.
Pursuant to the assumption of the decision problem, the analyzed population are adult patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression. Therefore, studies which assessed patients below 18 years-of-age or with diseases such as gastrointestinal stromal tumor, metastatic renal cell carcinoma and other were excluded.
Pursuant to the Summary of Product Characteristic of Sutent® [5], sunitinib in the indication specified above should be given in the dose of 37.5 mg once a day, orally (continuous regimen). Therefore, all clinical trials assessing sunitinib given in the dose of 12.5 mg/d, 25 mg/d or 50 mg/d were excluded from this analysis.
Authors of this systematic review did not include in the main analysis publications available only in the form of abstracts or conferences reports due to the absence of a possibility of carrying out an assessment of reliability of this type of survey. The review covered all found randomized studies satisfying the predefined analysis inclusion criteria.
The comparator of the intervention assessed should be a valid practice [7]. Authors of the report consulted the selection of the comparator with a medical expert. Based on information about unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression treatment standards in Poland and the opinion of the medical expert, best supportive care was considered as the proper comparator for the intervention assessed.
To sum up, the systematic review and cost-utility analysis of sunitinib is consistent with the assumptions presented in the analysis of the decision-making problem. Population included in the analysis is consistent with the population included in the Summary of Product Characteristic of Sutent® [1]. The treatment period and chosen endpoints seems to be justified and sufficient to fully prove the efficacy of the intervention assessed.
Following the search of publications, one primary randomized clinical trial (phase III) was found (subtype II A) to satisfy the inclusion criteria for the analysis [4, 9, 10, 11, 12, 13]. The study directly compared the clinical effectiveness of sunitinib and BSC versus placebo and BSC in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression.
Sunitinib together with BSC in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression provides higher clinical efficacy and a comparable safety profile against BSC. Statistically significant differences to the benefit of sunitinib were found in respect of main endpoints: progression free survival and overall survival as well as the objective response rate. This clinical effectiveness analysis also showed statistically significant effects of sunitinib regarding the amount of total deaths and discontinuation from the study due to progression or relapse.
Inclusion of patients in the studies is based on clearly defined criteria, which are often rigorous. Such criteria must be examined before extrapolating studies results on to the general population. For this reason, it is essential to assess the similarity between the population surveyed and the target population, taking into account the clinical and demographic features of patients. The trial population (Raymond 2011) was relatively well-balanced, unselected, with demographic characteristics and treatment history that are typical for patients with advanced pNETs. On the other hand, rigorous criteria of including patients into the analyzed clinical trial (Raymond 2011) decreased the possibility of incorporating the results obtained into everyday clinical practice.
The QALY parameter was the measure of effectiveness in the CUA, which was calculated on the basis of modeling conducted using TreeAge Pro 2009. Similarly to the majority of economic analyses concerning profitability of other cancer treatment, a Markov model was implemented. The economic model predicted a gain of 0.98 QALYs from 0.46 QALYs in the BSC arm to 1.44 with sunitinib. This is a very substantial increase for those patients.
Results of the cost-utility analysis proved that a therapy containing SUN+BSC is more expensive and more effective than BSC alone. Treatment with the use of Sutent® leads to extending overall survival (OS) as well as time to progression (TTP).
According to the recommendations of the consultation board of the Agency for Health Technology Assessment in Poland concerning the threshold of medical technologies profitability, sunitinib therapy with BSC in the treatment of pNETs is a cost effective strategy in comparison with BSC alone when the measure of effectiveness is QALY. ICUR is below the acceptability threshold in Poland (which is about PLN 99,543).
At present, patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression do not have any other effective treatment option. A positive reimbursement decision for sunitinib will increase the possibilities of treatment in this group of patients.
Conclusions
These analyses suggest a survival advantage and further support the clinical benefit of sunitinib for adult patients with progressive unresectable or metastatic
well-differentiated pancreatic NETs.
The results of the present clinical effectiveness analysis clearly prove that sunitinib administered in a 37.5 mg dose is an effective and safe therapy in the treatment of patients with unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors with disease progression. Sunitinib in combination with BSC prolongs overall survival and time to next progression.
The reimbursement of sunitinib would bring benefits to patients for whom there is currently no other effective treatment option. Compared with BSC, sunitinib treatment in patients with advanced pNETs improved effectiveness in terms of QALYs gained and the ICUR was within the range of what is considered cost-effective in Poland.