Influenza – the greatest master of metamorphosis – constant puzzle
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Flu decimated the human population from time immemorial. It was and still continues to be the cause of many human tragedies. Statement by Dr Kevin Sullivan of the USA quoted Influenza is defined as an uncontrolled scourge of humanity remains constantly up to date, because of our irreverent relation to the fight against this pathogen [1]. According to information from WHO, between 330 million and 1.575 billion individuals suffer from influenza and influenza – like virus every year throughout the world, with deaths of between 0.5 and 1 million individuals [2].
Influenza is a disease in which the continuous evolution of the virus is essential for the occurrence in the human population with seasonal epidemic, and from time to time also pandemic. For example, 9 may 1997, the avian influenza virus A/H5N1/ broke the barrier of species and became pathogenic to humans [3]. Almost 60% of people infected with A/H5N1/ die, and therefore was labeled as Highly Pathogenic Avian Influenza (HPAI). Influenza A virus infects not only humans, but also horses, pigs, minks, aquatic mammals such as seals, whales, and birds especially aquatic birds. The all subtypes were found among birds, but only a few of them also among the people [5,6].
In the peer for years, the number of influenza virus hemagglutinins HA [H1-18] and neuraminidase NA of influenza virus NA [N11] is increasing due to the isolation of influenza virus from some species of bats [7,8]. The evolution of the influenza virus is most apparent in the case of surface glycoproteins of the virus, but it also relates to each of the eight viral gene segments, both type A and B. This difference results from the accumulation of the molecular changes in the eight RNA segments that may occur by various mechanisms, which may contribute to the evolution of influenza viruses [5,6].
Modular construction of the genome of influenza virus is also responsible for the huge variation in both genotype and phenotype. The ancient Greek philosopher who lived in 540 – 480 BC, Heraclitus of Ephesus wrote: Nothing is permanent except change, and in the case of influenza virus works. In the XX century there were three pandemics: 1918-19 - Spanish flu – A/H1N1/ 50-100mln caused the victims death, Asian flu -A/H2N2/, caused 1-4 million victims of death, Hong Kong flu -A/H3N2/ led to 1-4 million victims of death. From this period in the epidemic season generally revolve two types of influenza virus type A (subtype A/H1N1 and subtype A/H3N2/) and type B [1,5,6].
The cornerstone, which caused the development of researchmultifaceted influenza virus was the isolation of influenza virus from humans by three British researchers Ch. Andrewes, W. Smith and P. Laidlaw at the National Institute for Medical Research in London. The next step is the multiplication of viruses in eggs in 1937 (which is currently used) resulted in the ability to produce influenza vaccine, as the only effective method of preventing illness, as well as post-influenza complications and death. In 1941, the first permit for the use of vaccines in humans. They were sponsored by the United States Armed Forces. In 1967 initially inactivated vaccine was purified containing the gradient of gravity. Then, in 1968, receiving the vaccine split and in 1976 subunit vaccine, in 1996 vaccine with oil adjuvant MF59, and in 1997 virosomal vaccine [1,2]. Next year resulted in various modifications of inactivated influenza vaccine as well as live-attenuated recommending influenza vaccine [LAIV] by ACIP in 2011, currently influenza virus strains used for the production of influenza vaccines. Thanks to the latest molecular biology techniques turn out to be almost 100% compatible with those that appear in the next epidemic season [9].
It's hard not to mention that in Poland in the years 1983-1984 in the National Centre for Influenza in the Department of Virology, National Institute of Hygiene in Warsaw in cooperation with the Military Institute of Epidemiology obtained inactivated influenza vaccine [1]. One of them was inactivated vaccine chromatographic purity, containing whole virions. The second one, which also had a purity of chromatographic, was vaccine subunit type, i.e. contain only the hemagglutinin and neuraminidase. These vaccines were obtained on a laboratory scale. Evaluation of the results of their application in animal and controlled human studies showed improved quality and effectiveness in comparison with conventional inactivated vaccine, manufactured by the Serum and Vaccine Production Plant in Krakow.
A total of 1,215 people were vaccinated, and made a comparative study of four different groups of people vaccinated three-component vaccine against influenza with different levels of purification, indicating both the level of antibodies antihaemagglutinin and antineuraminidase. The resulting vaccine had called a state control. Was tested by the Department of Sera and Vaccines at the National Institute of Hygiene in Warsaw. The results of these studies suggested the possibility of production of modern vaccines do not deviate from the standards of the World Health Organization. The authors of this study was three-person team: Professor Lidia B. Brydak Ph.D., Professor Wiesław Gall MD., and not living now Romuald Semkow MD. This work was pioneering in Poland regret it should be noted that the development is not implemented for mass produced. It is the only development of technology vaccine against influenza in Poland [10]. In 1980-1990 the research was conducted on the adaptation of influenza viruses A/H3N2/ antigenic formula for low temperature replication, and then analyze their antigenic and biochemical characteristics necessary to determine the possibility of using them as donor genes using recombinant method for producing vaccine strains. Such designation indicators - genetic markers necessary for this type of research for output strains and cold adapted, that is adapted to low temperatures. As a result of these studies yielded two polish mutants A/Pol/L/71 / H3N2/ and A/Pol/79/85 which can be used as donor genes using a method for producing recombinant influenza vaccine strains, as was confirmed by Professor L. Döhner Ph.D., of the University of Greifswald in Germany. This work was the subject of dessertation thesis of Professor Lidia B. Brydak PhD. For several epidemical influenza seasons doctors of all specialites have to the disposal many kinds of influenza vaccines, ranging from different types of inactivated - to live-attenuated influenza vaccine (LAIV) produced in eggs and various tissue culture.
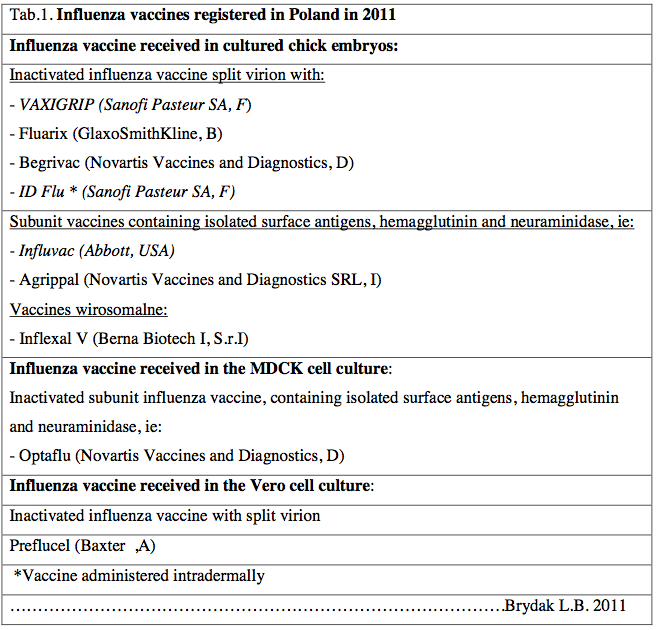
Table 1 shows the vaccine registered in Poland. In the epidemics seasonal 2013/2014 are available vaccine written by slash.
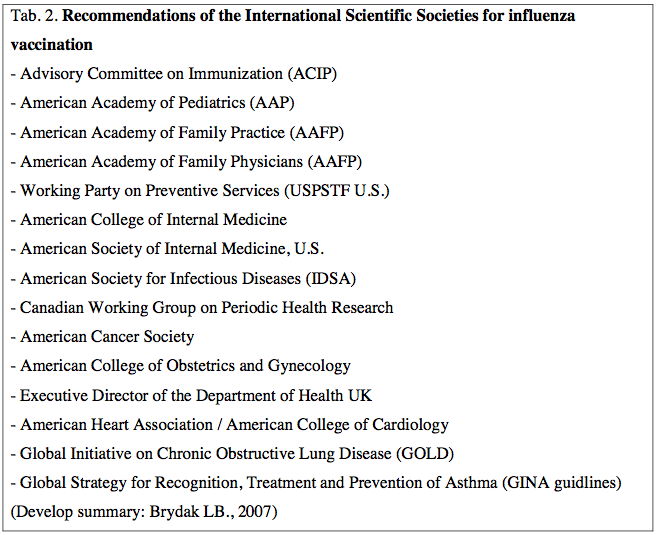
According to the Recommendations of the Advisory Committee on Immunization Practices (ACIP) recommended vaccination should we all willing ranging from 6th month until the ripe old age and instill the highest percentage of the population in each country [2.12]. Vaccinated against influenza we should due to clinical indications and epidemiological indication not only ACIP recommends vaccination against influenza but also 14 International Scientific Societies as shown in Table 2.[1].
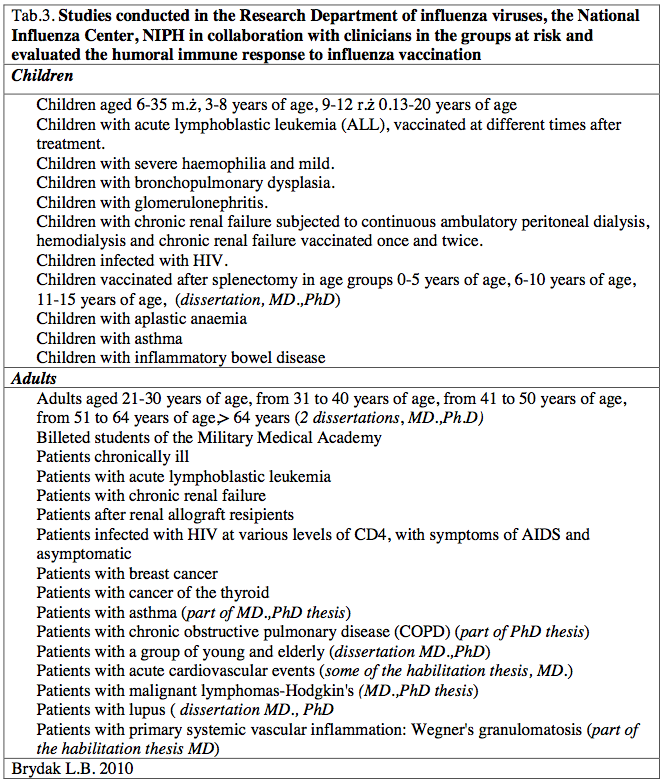
As Director of the National Influenza Center, Head of the Department of influenza Research at the National Institute of Public Health-National Institute of Hygiene in Warsaw in the nineties to the present time I have started working with clinicians concerning research on the immune response after vaccination with influenza-risk children and adult patients resulted in numerous publications in journals of national, international, reports at international congresses and conferences of national [14-34]. These studies were designed to increase influenza vaccination, evaluation of the humoral response and to provide specific examples will be helpful in promoting molding and encourage health professionals to protect not only patients but also their loved ones. As a result to carry out research in the following high-risk groups [Table 3].
Table 3. Studies conducted in the Research Department of influenza viruses, the National Influenza Center, NIPH in collaboration with clinicians in the groups at risk and evaluated the humoral immune response to influenza vaccination
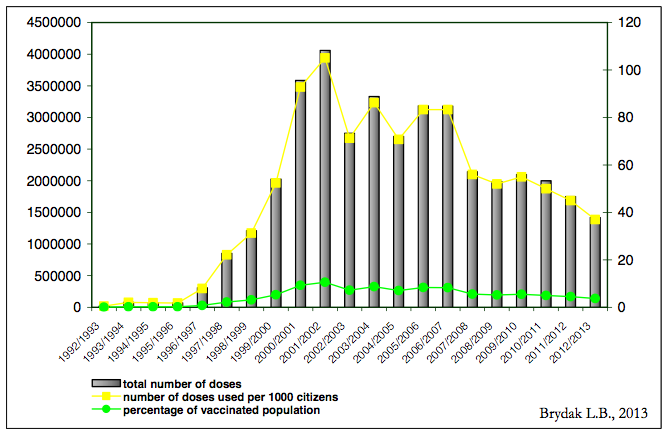
The results of our studies of influenza vaccination in patients with acute cardiovascular events were included in the recommendations of the European Cardiac Influenza Vaccination [12]. There are hundreds of clinical studies published in reputable scientific journals documenting that influenza vaccination prevent multisystem post-influenza complications, I regret to say that Poland is on the penultimate place in Europe regarding the percentage of vaccinated population [2]. The epidemic season 2012/2013 instilled only 3.75% of the population, in spite of that in Poland for many influenza seasons in many regions local authorities allocate a certain amount of funds to offer free flu vaccination to people 50 – 65 years of age who are not once in the high-risk group. Figure 1 shows the percentage of the population vaccinated against influenza in Poland [13].
Figure 1. Usage of inactivated vaccine against influenza in Poland in the epidemic season 1992/1993 – 2012/2013
The age range which offers a free flu vaccination depends only on the decision of Health and Social Affairs of the City and is different depending on the region. According to the Act of 5 December 2008, Article 51 of the Act of 5 December 2008 on preventing and combating infections and infectious diseases in humans - Acts. U. No. 234, item. 1570, which entered into force dn.1 January 2009, the doctor is obliged to inform the patient about resulting from the provision of the Act, requiring a protective vaccinations listed in the relevant ordinances. Failure to comply with this requirement may result in legal consequences (adjudication shall follow the provisions of the Act dated. August 24, 2001 - Code of Conduct misdemeanor cases Coll. Laws of 2008, No. 133 and No. 214 poz.848, item. 1344).
Please be aware that multi-organ post-influenza complications due to influenza infection may occur from persons at high risk, pregnant women, infants, small children of the adults and the elderly, and also in young people who were previously healthy. Multi-organ post-influenza complications often should also be considered in terms of human tragedy is not computable for money and very serious economic costs incurred by our Country.
Already in 2008, I suggested to develop and implement the National Programme for Prevention of Influenza in order to protect the population against the Polish extremely dangerous pathogen. I described it in my book: FLU, flu pandemic myth or real threat? in the chapter on public health (pp. 437-465). In 2012, the Working Group was established Influenza and developed such a program, which now has the name of National Programme for Influenza Prevention and was presented at the conference Vaccinations in NIPH-NIH on 16 April 2013 is available on the websites of www.opzg.pl. According to studies, Ernst & Young, which is contained in the National Programme for Influenza Prevention and Influenza costs in Poland should take into account the direct costs of treatment and indirect costs of influenza. The direct costs of treatment of influenza: ie expenditure on drugs, visits to doctors, treatment of post-influenza complications, specialized tests, hospitalizations, and represent only a small part of the total costs borne by society as a result of illness from the flu and its complications, and is approximately PLN 730 million in the year epidemic [approximately PLN 43.5 million a year without epidemic].
Indirect cost: that reflect the loss suffered by the economy as a result of staff sickness absence or long-term absence from work due to illness or caring for the sick, the decline in labor productivity sick, but not being on sick leave, severe post-influenza complications, which can lead to partial or total incapacity, severe post-influenza complications, which can lead to the death of the employee. The indirect costs of influenza in Poland amount to approximately PLN 4.3 billion in the year of the epidemic [approximately PLN 836 million a year without an epidemic].
Costs difficult to classify in economic terms: for example, reduced quality of life due to pain, loss of free time, reducing the possibility of functioning such as social activity.
Marcus Aurelius, Roman emperor who lived from 121-180, said: Man is worth so much, how many things are matters coming before it. These words are also valid in the XXI century.
Corresponding author:
Professor Lidia B. Brydak Ph.D
Director of National Influenza Centre, Head of the Department of Influenza Research
National Institute of Public Health-National Institute of Hygiene
00-791 Warsaw. Chocimska street 24
Phone no./fax.: 48 22 54 21 313
email: l.brydak@pzh.gov.pl
- Brydak, LB. Influenza, pandemic flu myth or a real threat? Rhythm, Warszawa 2008; 1- 492 [in Polish]
- Available from: www.who.int
- Webster RG. Origin of epidemic and pandemic influenza viruses.35th Annual Meeting of the Infectious Diseases Society of America. San Francisco 13-16 September 1997, USA
- Dan Zheng., Yinglei Yi, Ze Chen. Development of live – attenuated influenza vaccines against outbreaks of H5N1 influenza. Viruses 2012; 4: 3589-3605
- Cox NJ., Subbarao K. Influenza. Lancet 1999; 353: 1277-82
- Webster W.G., Bean WI., Gorman OT., Chamers TM., Kawaoka Y. Evolution and Ecology of Influenza A Viruses. Microbiology Reviews 1992; 56:152-179
- Xiaoman Sun., Yi Shi , Xishan Lu, Jianhua Gao, Jonghua Yan, Jianxun Qi, and George F.Gao. Bat-derived influenza hemagglutinin H17 Does bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Reports 2013; 3: 760-778
- Qing Li, Xiaoman Sun, Zhixin Li, Yue Liu, Vavricja JCh., Kainxun Qi et al. Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus. Proc Natl Acad Sci, 2012; 109,26: 18897-18902
- Weekly Prevention and Control of Influenza Recommendations of the Advisory Committee on Immunization Practices (ACIP). 2011. Center for Disease Control and Prevention, Morbidity and Mortality Weekly Report MMWR CDC Surveillance Summarie. Atlanta, GA; 10: 815
- Brydak LB., Gall W., Semkow R. Comperative anti-influenza vaccination of some groups of the population with vaccine differing in virus purification level. Arch Immunol et Ther Exp 1987; 35: 201-206
- Brydak LB. Characteristic of influenza viruses A/H3N2/ adapted to low temperature replication. 1-135 [ in polish, National Institute of Hygiene Warszawa]
- Brydak LB. Influenza – the passion of my academic research life. Top Medical Trens. Przewodnik lekarza 2011; 1: 19-24
- Brydak LB. Commentary on the article: Participation of senior citizen of the city of Poznań in gratuitous preventive vaccination against influenza. Probl. Hig. Epidemiol; 94(4): 2-3
- Brydak LB., Białek J., Rudnicka H., Denys A., Renery H., Cox N. 1997. Seroconversion assessment in billeted Military Medical University student group after antiinfluenza subunit vaccinations in 1993/1994 in Poland. Antiinfective Drugs and Chemotherapy; 15: 13-16
- Brydak LB., Całbecka M. Immunogenicity of influenza vaccine in patients with hemato-oncological disorders. Leuk Lymphoma 1999; 32: 369-374
- Brydak LB., Frącka B., Maruszak M., Rudnicka H., Nachman SA. Influenza immunization for children with bronchopulmonary dysplasia in Poland. Pediatr Infect Dis 1997; 16: 538-539
- Brydak LB., Guzy J., Starzyk J., Machała M., Góźdź S. Humoral immune response after vaccination against influenza in patients with breast cancer. Suppor Care Cancer 2001; 9: 65-68
- Brydak LB., Hryniewicz HJ., Machała M., Horban A. Humoral response to influenza vaccination in HIV-infected patients. Clinical Drug Investigation 1999; 17: 441-449
- Brydak LB., Machała M., Bentkowski P., Warzocha K., Biliński P. Humoral response to influenza vaccination in patents with non-Hodgkin lymphoma. Vaccine 2006; 24: 6620-6623
- Brydak LB., Machała M., Łaguna P., Rokicka-Milewska R. Antibody response to hemagglutinin and neuraminidase in splenectomized patens vaccinated against influenza in Poland. Clin Infect Dis 2004; 24: 225-236
- Brydak LB., Machała M., Myśliwska J., Myśliwski A., Grzonkowski P. Immune response to influenza vaccination in an elderly population. J of Clin Immunol 2003; 23: 214-222
- Brydak LB., Rokicka-Milewska R., Jackowska T., Rudnicka H., Renery H., Cox N. Kinetics of humoral response in children with acute lymphoblastic leukemia immunized with influenza vaccine in 1993 in Poland. Leuk Lymphoma 1997; 163:169-174
- Brydak LB., Rokicka-Milewska R., Klukowska A., Rudnicka H., Renery H., Cox N. Antibody kinetics in children with hemophilia immunized with influenza vaccine in 1993 in Poland. International Journal of Pediatric Hematology/ Oncology 1998; 5: 13-19
- Brydak LB., Rokicka-Milewska R., Machała M., Jackowska T., Sikorska-Fic B. Immunogenicity of subunit trivalent influenza vaccine in children with acute lymphoblastic leukemia. Pediatr Infect Dis J 1998; 17: 125-129
- Brydak LB., Roszkowska-Blaim M., Machała M., Leszczyńska B., Sieniawska M. Antibody response to influenza immunization in two consecutive epidemic seasons in patients with renal diseases. Vaccine 2000; 18: 3280-3286
- Brydak LB., Skwarczyński T., Machała M. Antibody response to influenza vaccination in healthy adults. Viral Immunol 2004; 17: 609-615
- Ciszewski A., Bilińska ZT., Brydak LB.,. Kepka C., Kruk M., Romanowska M., Księżycka E., Przyłuski J., Piotrowski W., Maczyńska R., Rużyłło W. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease. FLUCAC study. Eur Heart J 2008; 29: 1350-80
- Jahnz-Różyk K., Brydak LB., Targowski T., Machała M., Płusa T. Effect of influenza vaccinations on immune response and serum eotaxin level in patients with allergic bronchial asthma. Mediators of Infammation 2004; 13: 195-9
- Kozioł-Montewka M., Książek A., Majdan M., Stasiewicz D., Brydak LB., Janicka L., Toś-Luty S., Sitkowska J., Skórska C., Ratoszyńska J., Przylepa E. Influence of some immune factors on the IL-6 and soluble IL-2 receptors in haemodialysed patients. Int Urol and Nephrol 1997; 29: 369-75
- Mastalerz-Migas A., Steciwko A., Brydak LB. Immune response to influenza vaccine in hemodialysis patients with chronic renal failure. Adv Exp Med Biol 2013; 756: 285-290
- Więsik-Szewczyk E., Romanowska M., Milenik P., Chwalińska-Sadowska H., Brydak LB., Olesińska M., Ząbek J. Anti-influenza vaccination in systemic lupus erythematosus patients: an analysis of specific humoral response and vaccination safety. Clin Rheumatol 2010; 29: 605-13
- Wyzgał J., Brydak LB., Zygier D., Pączek L., Rowiński W., Grochowiecki T. Study on efficacy of influenza vaccination in renal allograft recipients. Transplantation Proceedings 2002; 34: 572-575
- Życińska K., Romanowska M., Nowak I., Rybicka K., Wardyn K., Brydak LB. Antibody response to inactivated subunit influenza vaccine in patients with Wegener’s granulomatosis. J Physiol Pharmacol Suppl 2007; 5: 819-828