Public-private collaboration to advance the development and benefit-risk assessment of vaccines: The Innovative Medicines Initiative
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Angela Wittelsberger |
Innovative Medicines Initiative Executive Office, Avenue de la Toison d\'Or 56-60, B-1060 Brussels |
|
Michel Goldman |
Innovative Medicines Initiative Executive Office, Avenue de la Toison d\'Or 56-60, B-1060 Brussels |
|
Wittelsberger Angela |
|
The true value of vaccines to public health remains to be fully exploited. Many of the challenges associated with vaccines, such as public distrust in the overall safety of vaccines, or finding standardised approaches to measure vaccine effectiveness, require the involvement of a number of different stakeholders in order to be successfully addressed.
Public-private partnerships such as the Innovative Medicines Initiative (IMI) provide a neutral platform that facilitates collaboration on an unprecedented scale between industry, academia, regulators and other actors in the field of vaccine research.Introduction
Vaccines are one of the most effective preventative health measures we have. Many infectious diseases that caused millions of deaths in the 20th century are either completely or close to eradication now. Infectious diseases like smallpox or poliomyelitis no longer pose a fatal threat to mankind. For other diseases, such as diphtheria, haemophilus influenza, measles, mumps, pertussis, rubella, and tetanus, the number of deaths per year has been drastically reduced. More than 70 vaccines have been licensed to date, for use against approximately 30 microbes, and there are many more in the development pipeline. The global vaccine market is estimated at $32.05 billion in 2013 and is expected to reach $84.44 billion by 2022 [1]. As of 2010, 79% of vaccines were still produced in Europe, 13% were produced in the US, and 8% were produced in Asia [2]. Half of the investment of Vaccine Europe members is still made in Europe [2], but a trend away from Europe and towards Asia is observed and feared to continue for both sales and investment.
Numerous initiatives have promoted global collaboration in vaccine development, including those by the Bill & Melinda Gates Foundation, the Global Alliance for Vaccines and Immunization (GAVI), the World Health Organisation (WHO), and public-private and product development partnerships. Furthermore, the past decades have seen significant advancement of novel technology and newer generations of vaccines [3]. In reverse vaccinology, genomic information of an organism leads to the identification of novel antigenic targets [4]. Gene-based delivery using DNA or viral vectors and prime-boost combinations have successfully been used to elicit enhanced immune responses [5-7]. Computational and technological advances in the capacity to study genes, proteins and cells have resulted in the field of systems vaccinology that aims at understanding the mechanisms by which vaccines stimulate immunity and at predicting the efficacy of vaccines [8].
Yet significant challenges are still associated with vaccines. Efforts are being made to shorten the time lag that has historically existed in the introduction of new vaccines between high- and low-income countries, but important gaps in vaccination coverage remain. WHO recently estimated that 1 in 5 of all children who die before the age of five, that is more than 1.5 million children, lose their lives to vaccine-preventable diseases [9]. Coverage gaps exist not only between countries, but also within countries. For example, in a recent study by WHO and UNICEF, some countries experience a measles vaccine coverage rate which is 58% higher for the richest fifth of the population than for the poorest fifth [10].
Furthermore, scientific challenges in novel vaccine development still exist. For viral pathogens, such as influenza viruses or malaria, antigenic variation poses a scientific conundrum in vaccine development. It is also becoming increasingly clear that environmental and genetic factors play a role in the effectiveness of vaccines. More personalised approaches to vaccination have therefore been proposed [11,12].
Finally, vaccines face a societal challenge – there is a real risk of low uptake due to perceived safety issues. Since vaccines are given to healthy people, safety is ranked high, and any scare about vaccine safety sensitively impairs public acceptance of immunisation programs, both in high-income and in lower-income countries. Accurate assessment of the link between vaccination and rare adverse events requires large sample sizes. The same is true for vaccine effectiveness. In addition, proper information systems are required for broader assessment of vaccine impact and vaccination coverage, as well as background distribution of adverse events, and the burden of preventable disease before and after vaccination was implemented.
To address these challenges, different stakeholders need to play a role. The vaccine industry is essential for their know-how in vaccine development and production; public health organisations are key for their epidemiology, surveillance data and recommendations on vaccination; small specialist companies and academic groups need to bring in novel technologies and knowledge management approaches. There is a need to align the decision-making of reimbursement agencies and national government vaccination committees with the development goals of the scientific community, as shows the recent example of the UK MenB case [13]. Successful collaboration among such a diverse set of stakeholders often requires the adoption of a different mind-set by the different parties, and this is why a neutral trusted platform is useful to facilitate exchange and interaction. The Innovative Medicines Initiative (IMI) offers such a platform with the facilitation of genuine partnerships between public and private players as one of its key missions.
Founded in 2007 by the European Union and the European Federation of Pharmaceutical Industries and Associations (EFPIA), IMI is the largest public-private partnership worldwide with the aim to facilitate the development of better and safer medicines for patients. With a total budget of €2 billion, 42 IMI projects are now up and running and a further 9 projects are in the preparatory phases. IMI projects have traditionally addressed bottlenecks in early phases of drug development, such as the identification and validation of novel biomarkers and models, or they deal with knowledge management and learnings that can be generated by pooling data. Some of the more recent IMI projects are of broader relevance to public health and deal with later phases in the drug development pipeline as well as post-market benefit-risk assessment [14].
Vaccination benefit-risk assessment
There is an increasing amount of data available on vaccine-preventable diseases, vaccination coverage and adverse reactions to vaccines, mostly due to a greater use of electronic health records, and robust infectious disease surveillance systems which are now in place. However, the information is currently largely fragmented into geographically-limited and non-standardised databases, and access to data is sometimes restricted.
The pioneering work under the Vaccine Safety Data Link project in the US [15] and the first European experiences gathered by the Vaccine Adverse Event Surveillance and Communication (VAESCO) and I-MOVE projects [16-18] have paved the way for a broader and sustainable, readily-available framework for combined benefit/risk measurements that are based on standardised, automated and validated processes.
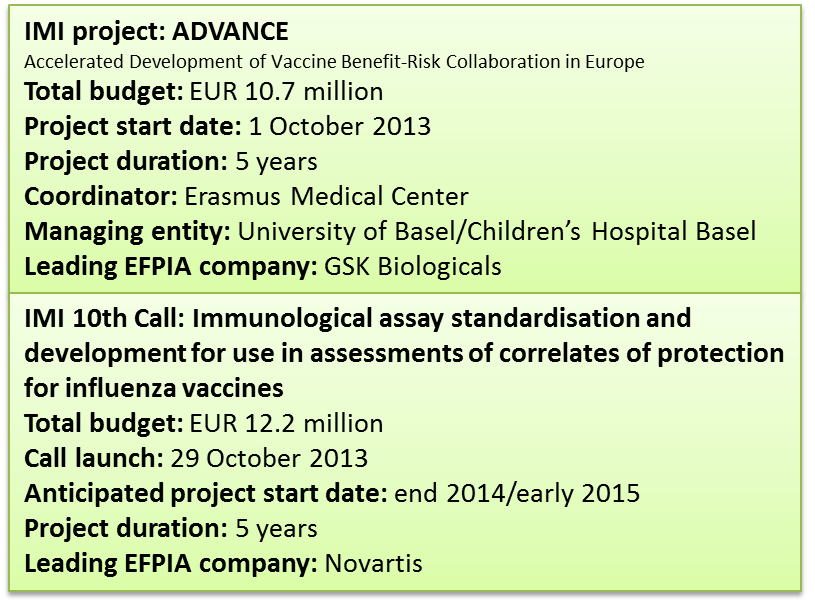
The IMI project ADVANCE launched in October 2013 in response to IMI’s 7the Call topic ‘Developing a framework for rapid assessment of vaccination benefit/risk in Europe’ recognises the need to be able to address concerns about the risk associated to vaccination in a timely and commonly accepted manner. The goal of ADVANCE is to review, develop and test methods, data sources and procedures which should feed into a blueprint of an efficient and sustainable pan-European framework that can rapidly deliver robust quantitative data for the assessment of vaccine benefits and risks. It is hoped that such a framework will allow regulators and public health authorities to make fast, informed decisions regarding vaccination strategies, and help to restore public confidence in vaccines. A key step in making this work will be to link up the data that is available in different places and different countries, in order to make it possible to analyse it. This step must resolve not only the inter-operability of the different data sources, but also the associated ethical and legal issues and variations thereof within different countries.
Of note, ADVANCE brings together different stakeholders as full partners, i.e. the vaccine developing industry, major public health and regulatory organisations including the European Center for Disease Prevention and Control (ECDC), the European Medicines Agency (EMA), a number of national public health and regulatory bodies, and academic experts in data mining and data linkage. In addition, the consortium is built around an open participation concept fostering contributions by institutions invited to contribute on an ad-hoc basis under a Memorandum of Understanding throughout the lifetime of the 5 year project but without having to officially adhere to the contractual framework established by the consortium partners at the beginning of the project. This is essential to ensure that the ultimate goal of the project, a blueprint of an efficient and sustainable vaccination benefit-risk assessment framework, will be implementable and acceptable to all stakeholders. One focus of the ADVANCE project will therefore be the definition of ‘best practice’ and a code of conduct, including the definition of rules for interactions between the public and private stakeholders.
The project will run a number of proof-of-concept studies to ensure the platform meets the needs of its users. In order to cover the most common situations, these studies should ideally cover different age groups (e.g. infants / children, adolescents and adults / the elderly), different risk groups (e.g. pregnant women, people with other underlying health problems), and different vaccination scenarios (e.g. annual flu vaccinations, or vaccines introduced into routine immunisation programmes).
In all aspects of its work, ADVANCE will exploit synergies with related projects. For example, the team will work closely with IMI’s EMIF project on data frameworks [19], and draw on the PROTECT project’s expertise in analysing and visualising the benefits and risks of medicines [20].
Development of influenza vaccines
While ADVANCE is dealing with challenges faced by vaccines post-licensure, clinical development of novel vaccines faces another set of difficulties. The effectiveness of vaccines needs to be demonstrated in clinical efficacy trials. For a pharmaceutical company, the process is often too costly, long and risky to provide sufficient incentives for engaging in vaccine development, in particular for diseases of the poor where limited return can be expected from low-income countries. Therefore, initiatives incentivising vaccine development have been warmly welcomed. For example, the European Vaccine Initiative (EVI) or the PATH Malaria Vaccine initiative have both helped advance novel malaria vaccine candidates with one novel vaccine each in later stages of clinical development [21].
As for influenza vaccines, the situation is complicated by the fact that an effective vaccine needs to be produced each and every year, based on the actual relevant viral strains identified. Vaccines developers produce the inactivated or life attenuated vaccine consisting of the strains of influenza virus recommended by the WHO. They run assays to analyse the effectiveness of a new vaccine, or to expand the use of existing vaccines to other age groups or categories. Public health and academic laboratories are involved in investigating how a vaccine performs in different target groups, and make recommendations on the ways the immunogenicity and efficacy of influenza vaccines should be evaluated.
The issue here is that there is no standardised correlate of protection and that the assays used to evaluate vaccines vary between laboratories. Each laboratory uses its own assay protocols that are reviewed every year to adapt to the change in strain. As a result, it is challenging to compare studies and to agree on the effectiveness of a vaccine.
Furthermore, due to the yearly change in strains, any effort to achieve standardisation must consider the question whether the priority should be to implement a strain-specific consensus protocol, an effort that will need to be repeated every year, or whether harmonisation of more generic procedures and standards should be prioritised.
The need for standardisation of serological assays is broadly recognised by public health, academic and industry investigators. The currently best validated and commonly used assay for regulatory submissions is the haemagglutination inhibition (HAI) assay. However, rigorous standardisation is lacking, and it is largely recognised that the HAI assay is inherently quite variable and labor-intensive. A higher throughput and more robust test would be very welcome. Another commonly-used test is the virus neutralisation assay (VN), but here too no standardised assay and protocol exists, nor was a clear correlate of protection ever established.
With the event of the 2009-2010 influenza pandemic, numerous efforts to optimise comparability and align interpretation of influenza serological studies have been put in place. For example, the Global Consortium to Standardize Influenza Seroepidemiology to Inform Public Health Policy (CONCISE) includes a number of public and government institutions working together with the aim of generating best practices for flu seroepidemiologic investigations.
The EMA is currently finalising new guidelines on influenza vaccines with the intention to develop a single, harmonised guidance for both seasonal and pandemic influenza vaccines. A draft concept paper issued in 2011 as well as several meetings and workshops leading to the revised guidelines highlighted the need for assay standardisation [22,23]. There have been international collaborative studies involving several laboratories to evaluate assay reproducibility, using candidate standard serum preparations or sera panels from clinical vaccine trials [24-28]. A recent collaborative effort by the Paul-Ehrlich Institute and the National Institute for Biological Standards and Control in association with the EMA analysed assay variability for the HAI and VN assays in different laboratories. A marked inter-laboratory variation of up to 5.8-fold for the HAI assay, and of up to 7.0-fold for the neutralisation titres was found [24]. Importantly, the variation was drastically reduced when calibrated antibody standards were used, indicating that the reproducibility of immunogenicity results can be improved through standardisation. The IMI held a consultation workshop prior to the launch of its 10th Call ‘Immunological assay standardisation and development for use in assessments of correlates of protection for influenza vaccines’. Since the objective of that workshop was to receive expert input for the optimisation of the IMI 10th Call topic, no formal report has been published, but many of the recommendations are reflected in the IMI 10th Call text, published online on 29 October 2013 [14]. All these studies and meetings resulted in the recommendation that further research was much needed into the standardisation of serological assays, on correlates of protection and how serology is predictive of vaccine efficacy, and on vaccine efficacy endpoints. Achieving standardisation of serological assays is considered a necessary first step in any effort towards clinical validation of a correlate of protection.
IMI’s 10th Call for proposals (deadline for submission of Expressions of Interest January 28, 2014) aims to address the need for standardisation of serological assays. The main focus of the €12.2 million effort is to achieve a common agreement on the way to perform HAI and VN assays, with the expectation that vaccine manufacturers, public health and regulatory laboratories all adhere to and implement the collaboratively-developed standardised protocols. In addition, work to advance our understanding and usefulness of less validated assays for the evaluation of influenza vaccine performance, such as cell-mediated immunity and NA assays, are invited in the Call. Although the scope of the current Call is standardisation of serological assays, it is anticipated that the existence and acceptance of standardised assays will then spur the establishment of correlates of protection to be tested in future clinical trials.
Concluding remarks
Vaccines have a great value to society, but it remains a challenge to fully exploit that. Innovative models of multi-stakeholder collaboration have arisen that bring together the vaccine industry, academic teams, regulatory bodies and public health institutions. Collaboration between these public and private groups is innovative and requires an open mind-set. A neutral platform such as the IMI helps to facilitate exchange and, as a result, should improve the general acceptance and impact of the project outcomes.
Disclaimer
The opinions expressed in this article do not necessarily reflect the positions and opinions of the European Commission or the EFPIA.
Acknowledgements
The authors wish to thank Sarah Black for her help in reviewing this manuscript.
- 1. RnRMarketResearch.com, Report released August 9, 2013. Available from: http://www.rnrmarketresearch.com/; [Accessed: 26.11.2013]
- 2. Available from: http://www.vaccineseurope.eu; [Accessed: 26.11.2013]
- 3. Nabel JG. Designing tomorrow’s vaccines. N. Engl. J. Med. 2013; 368: 551-560
- 4. Kelly DF., Rappuoli R. Reverse vaccinology and vaccines for serogroup B Neiseria meningitides. Adv. Exp. Med. Biol. 2005; 568: 217-223
- 5. Mascola JR., Sambor A., Beaudry K., et al. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J. Virol. 2005; 79: 771-779
- 6. Wang S., Kennedy JS., West K., et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 2008; 26: 3947-3957
- 7. Wei CJ., Boyington JC., McTamney PM., et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 2010; 329: 1060-1064
- 8. Nakaya HI., Li S., Pulendran B. Systems vaccinology: learning to compute the behavior of vaccined induced immunity. Rev. Syst. Biol. Med. 2012; 4: 193-205
- 9. Child Health Epidemiology Reference Group of WHO and UNICEF. Black R.E., Cousens S., Johnson HL., et al.: Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969-1987
- 10. GVAP document. Available from: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/; [Accessed: 26.11.2013]
- 11. Poland GA., Ovsyannikova IG., Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics 2009; 10: 837-852
- 12. Moxon ER., Siegrist CA. The next decades of vaccines: societal and scientific challenges. The Lancet 2011; 378: 348-359
- 13. Mekalanos JJ. Vaccine economics: What price human life? Sci. Transl. Med. 2013; 5: 204ed16
- 14. Available from: www.imi.europa.eu; [Accessed: 26.11.2013]
- 15. Baggs J., Gee J., Lewis E., et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011; 127: S45-S53
- 16. The VAESCO case-control study group. Dieleman J., Romio S., Johansen K., et al.: Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ 2011; 343: d3908
- 17. The VAESCO consortium. Andrews N., Stowe J., Miller E., et al.: A collaborative approach to investigating the risk of thrombocytopenic purpura after measles-mumps-rubella vaccination in England and Denmark.Vaccine 2012; 30: 3042-3046
- 18. Kissling E., Larrauri A., Bella A., et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro surveillance : bulletin Européen sur les maladies transmissibles - European communicable disease bulletin 2013; 18: 1-10
- 19. Available from: www.emif.eu; [Accessed: 26.11.2013]
- 20. Available from: www.imi-protect.eu; [Acceseed: 26.11.2013]
- 21. The RTS,S Clinical Trials Partnership, A Phase 3 Trial of RTS,C/AS01 Malaria Vaccine in African Infants, N Engl J Med 2012; 367: 2284-2295
- 22. EMA/CHMP/VWP/734330/2011
- 23. Laurie KL., Huston P., Riley S., et al. Influenza serological studies to inform public health action: best practices to optimise timing, quality and reporting. Influenza Other Respi. Viruses 2012; 1750-2659
- 24. Wagner R., Göpfert C., Hammann J., et al. Enhancing the reproducibility of serological methods used to evaluate immunogenicity of pandemic H1N1 influenza vaccines – An effective EU regulatory approach. Vaccine 2012; 30: 4113-4122
- 25. Stephenson I., Das R., Wood J., et al. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: An international collaborative study. Vaccine 2007; 25: 4056-4063
- 26. Stephenson I., Heath A., Major D., et al. Reproducibility of serologic assays for influenza virus A (H5N1). Emerging Infectious Diseases 2009; 15: 1250-1259
- 27. Wood J., Major D., Heath A., et al. Reproducibility of serologic assays for pandemic influenza H1N1: collaborative study to evaluate a candidate WHO international standard. Vaccine 2012; 30: 210-217
- 28. Wood JM., Montomoli E., Newman RW., et al. Collaborative study on influenza vaccine clinical trial serology – part 2: reproducibility study. Pharmeuropa bio & scientific notes 2011; 1: 36-54