Cost-effectiveness analysis of Human Papillomavirus (HPV) vaccination using Cervarix® as an extension to the cervical cancer prevention programme in Poland
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Katarzyna Wepsięć |
GSK Services Sp. z o.o., Warszawa, Poland |
|
Magdalena Mrożek-Gąsiorowska |
Pracownia HTA, Kraków, Poland |
|
Marcin Gąsiorowski |
Pracownia HTA, Kraków, Poland |
|
Oskar Pankiewicz |
Pracownia HTA, Kraków, Poland |
|
Andrzej Nowakowski |
Department of Gynaecology and Gynaecological Oncology, Military Institute of Medicine, Ministry of Defence, Central Teaching Hospital, Warszawa, Poland |
Background: The aim of the study was to estimate the cost-effectiveness of addition of human papillomavirus (HPV) vaccination to the Polish cervical cancer prevention programme.
Methods: A cost-utility analysis was conducted. A lifetime Markov model, adapted to Polish settings, was used to compare the costs and health outcomes of the two strategies, i.e. the existing cervical cancer prevention programme with or without universal HPV vaccination in girls at the age of 14.
Results: Assuming that the whole cohort was vaccinated (100% vaccination coverage), the estimated lifetime risk of developing cervical cancer would be reduced from 0.95% to 0.23%; therefore, 1311 cases of cervical cancer and 681 deaths due to cervical cancer would be prevented in a cohort of 182,000 girls aged 14 years.If the assumed vaccination coverage was 24%, the cost of gaining an additional quality-adjusted life year (QALY) due to HPV vaccination as an extension of the cervical cancer prevention programme would be PLN 52,737.91/QALY and PLN 76,288.47/QALY from the public payer’s perspective and the common perspective of the public payer and the patient, respectively.This cost effectiveness is maintained for different parameter assumptions in the sensitivity analysis. Even with high assumed discount rates for costs and health outcomes (5% for both), the ICUR value was still lower than the cost-effectiveness threshold (PLN 105,801 per QALY).
Conclusion: Addition of HPV vaccination to the cervical cancer prevention programme in Poland is a highly cost-effective intervention.
Introduction
Primary cervical cancer constitutes a vast majority of cases of uterine cancer and develops over many years from precancerous lesions known as cervical intraepithelial neoplasia (CIN) [1]. Depending on its histopathological features, CIN is currently classified into three cathegories, i.e.CIN1, CIN2, or CIN3. In Poland, cervical cancer is one of the most common cancers in women, and the absolute number of new cases and deaths due to this neoplasm in 2010 in Poland was 3078 and 1735, respectively; this was equal to the standardised incidence and mortality rates of 10.3 and 5.1/100,000 women, respectively [2]. The epidemiology of CIN in Poland is not accurately known. According to the National Cancer Registry, there were 775 cases of pre-invasive cervical cancer (currently classified along with CIN3) in 2010; however, this number is significantly underestimated because the reporting or registering of precancerous lesions is not mandatory in Poland. Carcinoma in situ/CIN3 is much more common than invasive cancer [3]. Pap smears enable early detection and treatment of precancerous cervical lesions; thus, they constitute the basis of screening programmes aimed at reduction of the cervical cancer incidence and mortality rates. However, introduction of an active screening programme in Poland in the years 2006/2007 did not result in increased dynamics of reduction of the cervical cancer incidence and mortality rates, mainly due to low coverage and unknown quality of the programme.
A necessary (although not sufficient) aetiological factor of cervical cancer is persistent infection with human papillomavirus (HPV) [1]. HPV is classified into high-risk types (hrHPV), i.e. those with a high carcinogenic potential (14 types: HPV-16/18/31/33/35/39/45/51/52/56/58/59/66/68) and low-risk types (lrHPV), e.g.HPV-6 or 11. Cervical cancer develops most commonly as a result of infection with HPV-16 or 18 which are responsible for more than 70% of all cases of cancer at that location, and, in addition, for a majority of high-grade intraepithelial precancerous lesions of the uterine cervix, vulva, vagina, anus, and penis [4,5]. At present, no anti-HPV medications are available on the market and therefore no causal treatment aimed at eradication of HPV is possible.Currently only treatment of histological abnormalities caused by HPV is possible and is most commonly based on its removal or destruction [6].
There are two HPV vaccines registered, i.e. Cervarix® (GSK) and Silgard® (MSD), and HPV vaccination programmes for women have been introduced in 19 countries in Europe. In most of these countries vaccination is completely financed from public resources [7]. According to the Polish Vaccination Programme (PVP) for the year 2013, HPV vaccination is recommended but not financed by the Ministry of Health from its budget [8]. In Poland, HPV vaccines are available on the market to patients but not reimbursed; they are also available locally in health programs introduced by regional government entities and other local authorities.
The aim of this analysis was to evaluate cost-effectiveness of Cervarix® in Poland if financed from public resources and used in prevention of cervical cancer and precancerous lesions associated with specific carcinogenic types of human papillomavirus. In this paper the results of a cost-utility analysis comparing the costs and outcomes of current practice in prevention of cervical cancer in Poland (i.e. a Pap smear performed every 3 years in women aged 25-59 years; the “Screening” strategy) with those of the same practice plus HPV vaccination using Cervarix® (the “Cervarix + Screening” strategy) are presented.
Materials and methods
The costs and outcomes associated with addition of HPV vaccination to the cervical cancer prevention programme in Poland were estimated based on a Markov cohort model developed by Demarteau et al., widely discussed in the literature, in which costs and outcomes of an intervention are assessed in a lifetime horizon – Global Cervarix Model version 12.0 (the model makes it possible to compare both a strategy including HPV vaccination vs. no vaccination and vaccination with Cervarix® vs. vaccination with Silgard®; therefore, data concerning genital warts were included in order to make potential comparison of costs and health outcomes of both registered vaccines possible) [9,10,11].This model has been adapted to Polish settings by means of inclusion of Polish epidemiological and cost data related to cervical cancer and CIN.
The analysis was performed from the public payer’s perspective and a common perspective of the public payer and the patient, assuming 30% patient’s co-payment for the HPV vaccine and partial coverage of the costs of treatment of genital warts by the patient (in Poland these costs are not completely covered by the public payer). In the base-case scenario, discount rates of 5% for the costs and 3.5% for health outcomes were assumed. The incremental cost-utility ratio (ICUR) was estimatedand cost-effectiveness of HPV vaccination was evaluated; the threshold value for one additional quality-adjusted life year gained was assumed at PLN 105,801, according to the guidelines of the Agency for Health Technology Assessment in Poland [12].
The effects of changes in the parameters assumed in the model on the results of the analysis were assessed using one-way sensitivity analysis and probabilistic analysis. The following parameters were taken into account: vaccination efficacy, the utilities of specific health states, the incidence and prevalence rates for specific HPV types in the population, costs of treatment of CIN, genital warts and cervical cancer, and the participation rate in screening.
Target population
In the model it was assumed that HPV vaccination would be administered to girls at the age of 14 years.Vaccination using a vaccine against type 16 and 18 human papillomavirus (Cervarix®) is indicated in individuals aged 9 years or more [13]. Depending on the country, national vaccination programmes include HPV vaccination in females aged 9-18 years. The Polish Gynaecological Society [14] and the Polish Paediatric Society [15] recommend basic HPV vaccination in girls aged 11-12 years and supplementary vaccination (catch-up programmes) in girls aged 13-18 years, while the Polish Society of HPV Infections Prophylaxis recommends basic HPV vaccination in girls aged 12-15 years and supplementary vaccination in young women aged 16-25/26 years [16]. HPV infection usually develops as a result of the first sexual contacts [17].Based on the results of a representative study conducted in Poland [18], the estimated sexual initiation rate in Polish girls below 15 years of age is very low. Target vaccination age of 14 years has been selected in order to maximize vaccination coverage of the cohort as a result of co-administration of Cervarix® with the booster vaccination againstdiphtheria and tetanus which, according to the PVP, is obligatory at this age [8].
Initial data
The health state utility values implemented in the economic model and data concerning the efficacy of cytological screening are presented in Table 1. Data concerning the efficacy of Cervarix® taken into account in the economic model were obtained from the PATRICIA study (in which the efficacy and safety of Cervarix® administered according to the 0, 1, 6 months schedule were compared with those of placebo [19,20,21,22,23]) or assumed based on the opinion of the experts involved in development of the model [9,10,11] (Table 2).
Table 1. Screening efficacy and utility values for specific health states included in the model
| Parameter | Value | Data source |
| Screening efficacy | ||
| CIN1 detection rate | 58% | [26] |
| CIN2/CIN3 detection rate | 61% | [26] |
| Proportion of positive Pap smears | 5.5% | [32] |
| Utilities (disutilities) | ||
| No HPV infection | 1 | [29, 31] |
| HPV infection | 1 | [29, 31] |
| Genital warts | (0.0180) | [35] |
| CIN1 detected | (0.0128) | [29, 31] |
| CIN2/CIN3 detected | (0.0094) | [29, 31] |
| Cervical cancer treated | (0.2730) | [29, 31] |
| Cervical cancer cured | (0.0620) | [29, 31] |
| Death | 0 | - |
Table 2. Efficacy of HPV vaccination and distribution of specific HPV types in Polish population
| Parameter | Distribution of HPV types – mean (range) |
Efficacy of Cervarix® – mean (range) |
| CIN1 | ||
| - HPV 16/18 | 23.9% (22.9%; 24.9%) [24] | 98% [21, 27, 28, 30, 33, 34] |
| - cross-protection | 45.6% (39.1%; 51.4%) [24] | 48% (29%; 62%) [21, 34] |
| - HPV 6/11 | 6.8% (5.6%; 8.0%) [24] | 0% |
| Overall effectiveness CIN1 | 65.2% | |
| Genital warts (GW) | ||
| - HPV 6/11 | 76.2% [25] | 0% |
| Overall effectiveness GW | 0% | |
| CIN2/CIN3 | ||
| - HPV 16/18 | 53.0% (51.9%; 54.1%) [24] | 98% [21, 27, 28, 30, 33, 34] |
| - cross-protection | 43.3% (42.2%; 44.1%) [24] | 68% [21,23] |
| Overall effectiveness CIN2/CIN3 | 81.4% | |
| Cervical cancer | ||
| - HPV 16/18 | 72.4% (67.5%; 77.1%) [24] | 98% [21, 27, 28, 30, 33, 34] |
| - cross-protection | 13.4% (5.4%; 26.5%) [24] | 68% (48%; 82%) [21,23] |
| Overall effectiveness cervical cancer | 80.1% | |
The target population size was estimated based on data published by the Central Statistical Office of Poland (CSO) and in the model the cohort size was assumed at 182,000 girls.
In the base-case scenario, taking into account data obtained from the Ministry of Health’s report on realisation of the National Cancer Control Programme for the year 2011 and the CSO data concerning the proportion of women who declare they had a pap smear performed in the previous 3 years, it was assumed that 49.29% of women took part in screening (either organised or opportunistic).
In the analysis it was assumed that 24% of the target population would be actually vaccinated.Such coverage was observed in 2008 in France, where 65% of the cost of HPV vaccination is reimbursed from public resources [7]. In addition, in order to evaluate the maximum health outcomes associated with introduction of HPV vaccination, a coverage level of 100% was assumed in a separate scenario [11].
Data used in the model included Polish epidemiological data concerning overall death rate (CSO data) and the number of new cases and deaths due to cervical cancer [2] as well as those concerning the prevalence of specific HPV types in the population and the HPV infection incidence rates, obtained from national registries and the World Health Organization database [24] (Table 2).
Direct medical costs incurred by the patient (i.e. patient’s co-payment for the HPV vaccine and costs of pharmacotherapy of genital warts) and the public payer (i.e. co‑financing of HPV vaccination and the costs of diagnostics and treatment of CIN and cervical cancer as well as those of surgical treatment of genital warts) were taken into account in the analysis. The average costs of diagnostics and treatment assumed in the model and the price of Cervarix® are presented in Table 3.
Table 3.Costs of diagnostics and treatment of specific health states
| Cost data | Cost [PLN] |
| Regular screening – women with negative Pap smear (including false negative results) | 53.87 |
| Management of CIN1 associated with hrHPV infection for one year after its detection | 964.53 |
| Further management after CIN1 has been cured | 53.87 |
| Management of CIN2 or CIN3 for one year after its detection | 2195.00 |
| Further management after CIN2 or CIN3 has been cured | 107.74 |
| Annual cost of treatment of cervical cancer | 5613.38 |
| Treatment of genital warts | 281.82 |
| Cervarix® - vaccine cost per injection [36] | 393.76 |
Results
The results obtained in the model indicate that addition of HPV vaccination using Cervarix® to prevention of cervical cancer in Poland is cost-effective. In a lifetime horizon, vaccination in girls aged 14 years was associated with additional health outcomes as expressed both in life years (LY)and quality-adjusted life years (QALY). Assuming a coverage level of 24%, the individual incremental value was 0.0036 QALY, which equals 657 additional QALY in the whole cohort of 182,000 girls.
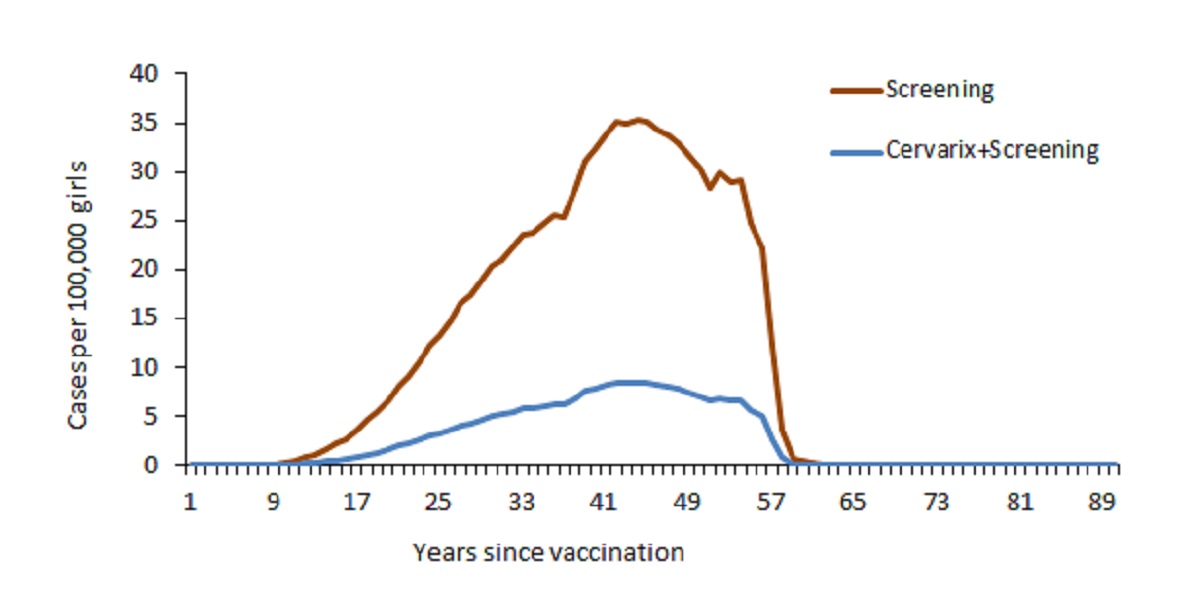
In comparison with cytological screening alone, addition of HPV vaccination (Cervarix®) was associated with increased efficacy with respect to prevention of CIN1 and CIN2/CIN3 as well as new cases and deaths due to cervical cancer. When a coverage level of 100% was assumed, HPV vaccination prevented 1311 cases of cervical cancer and 681 deaths due to cervical cancer (Figure 1) as well as 33,313 cases of CIN1 and 6791 cases of CIN2/CIN3 in the cohort’s lifetime. As expected, the “Cervarix + Screening” strategy did not prevent development of genital warts (Cervarix® is not indicated in prevention of this condition).
Regardless of the perspective adopted, the strategy including cytological screening and HPV vaccination in comparison with the strategy based on cytological screening alone was a cost-effective intervention (Table 4).
Table 4.Results of the economic analysis
| Category | Cervarix® + Screening | Screening | Incremental outcomes |
| Health outcomes: Quality-adjusted life years (QALYs) |
26.2836 | 26.2800 | 0.0036 |
| Total costs [PLN] (public payer)& | 409.02 | 218.56 | 190.46 |
| Total costs [PLN] (public payer and patient)& | 497.05 | 221.54 | 275.51 |
| including: | |||
| - HPV vaccination | 283.51 | 0.00 | 283.51 |
| - Treatment of CIN1 | 24.55 | 26.48 | -1.93 |
| - Treatment of CIN2/CIN3 | 7.42 | 8.85 | -1.43 |
| - Treatment of cervical cancer | 26.63 | 31.65 | -5.02 |
| - Treatment of genital warts | 9.79 | 9.79 | 0.00 |
| - Screening | 145.15 | 144.77 | 0.38 |
| Incremental cost-utility ratio (ICUR)# | |||
| - Public payer | PLN 52,737.91 | ||
| - Public payer and patient | PLN 76,288.47 | ||
& - Health outcomes and costs per patient in the modelled cohort (discounted values).
# - The ratio of the difference in costs to the difference of health outcomes of both interventions.
Conclusions
The analysis demonstrated that a strategy assuming addition of HPV vaccination using Cervarix® in girls aged 14 years to current cervical cancer prevention practice in Poland (i.e. Pap smears in women aged 25-59 years in a reimbursed screening programme) is a highly cost-effective intervention in comparison with current practice. Cervarix® prevents a higher number of precancerous lesions and cervical cancer cases as well as deaths due to cervical cancer than screening alone.
The results of this analysis indicated that, in comparison with current practice, a strategy including vaccination with Cervarix® in a cohort of girls aged 14 years and assuming a coverage level of 24% would make it possible to reduce both the incidence of cervical cancer and mortality due to cervical cancer by 16%. If the coverage level was 100%, the “Cervarix + Screening” strategy would make it possible to reduce both the incidence and mortality rate by 76%.
The analysis demonstrated that the cost of additional quality-adjusted life year gained (i.e. the ICUR value) for addition of HPV vaccination to the current cervical cancer prevention programme versus current practice alone was much lower than the recommended cost-effectiveness threshold in Poland (i.e. PLN 105,801 at present), regardless of the adopted perspective of the analysis. Financing of Cervarix® from public resources would make it possible to reduce the incidence of cervical intraepithelial neoplasia as well as the incidence and mortality due to cervical cancer, which in turn would reduce the costs associated with treatment of those conditions, in Poland at present amounting to nearly PLN 70 million annually.
Discussion
Numerous economic analyses conducted in other countries indicate that HPV vaccination performed in parallel with cytological screening constitute an efficacious and cost-effective strategy [37,38,39,40,41,42].
The benefits would be highest if all teenage girls were vaccinated; however, experience gained in the countries in which vaccination is partially reimbursed by the public payer indicate that it is not possible, at least in the first few years after introduction of reimbursement, to obtain such a high vaccination coverage. According to the opinion of Polish experts, in Poland the expected coverage rate in girls aged 14 years may be 10-15% in the first year of reimbursement of HPV vaccination. In subsequent years the coverage level may increase to 25-30% [43].
Evaluation of economic aspects of HPV vaccination is relatively difficult. This is due to the specificity of HPV infection and the fact that its consequences (especially cancerous lesions) develop even decades after the infection. Epidemiological data concerning HPV infection indicate that most sexually active women and men were, are, or will be infected with this virus. Introduction of universal vaccination against HPV, despite significant expenses in the first stage of a woman’s life, may result in savings due to a lower risk of CIN and cervical cancer in later stages. Taking into account that in most cases women are diagnosed with cervical cancer at the age of 50-60 years, HPV vaccination may also result in lower productivity loss.
Primary prevention of cervical cancer with vaccination may contribute to nearly complete elimination of the problem of mortality due to cervical cancer in Poland and therefore limit the number of orphaned families. Intangible costs associated with the disease cannot be estimated; however, epidemiological data, i.e. more than 3000 new cases and nearly 2000 deaths each year, demonstrate the scale of the problem affecting thousands of families and “wiping out” a population equivalent to a small town every few years. Therefore, reimbursement of HPV vaccination may be an important step towards a change of this situation.
Conflict of interests / sources of financing:
MMG, MG, and OP received remuneration from GlaxoSmithKline for development of a HTA report and publications concerning Cervarix®.
- Urbański K., Kornafel J., Bidziński M. et al. Zalecenia postępowania diagnostyczno-terapeutycznego w nowotworach złośliwych u dorosłych; Krzakowski M., Via Medica, Gdańsk 2009; 234-242
- Raporty na podstawie danych Centrum Onkologii. Available from: http://epid.coi.waw.pl/krn/; [Accessed: 11 April 2013]
- Cancer Research UK. Cervical screening – results. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/ cervix/screening/cervicalscreeningresults/cervical-screening-programme-results; [Accessed: 10 October 2013]
- de Villiers EM., Fauquet C., Broker TR., Bernard HU., zur Hausen H. Classification of papillomaviruses. Virology 2004 Jun 20; 324(1): 17-27
- Hirnle L. Zakażenia wirusami HPV – problem medyczny i społeczny. Gin Prakt 2009; 4: 8-12
- Macioch T., Niewada M., Wierzba W., Bidziński M., Radowicki S. Zapobieganie chorobom zależnym od zakażenia HPV – aspekty kliniczne i ekonomiczne stosowania szczepień profilaktycznych. Curr Gynecol Oncol 2010; 8(2): 69-81
- European Centre for Disease Prevention and Control. Introduction of HPV vaccines in EU countries – an update. Stockholm: ECDC; 2012. Available from: http://ecdc.europa.eu/en/publications/publications/20120905_gui_hpv_vaccine_update.pdf; [Accessed: 11 April 2013]
- Komunikat Głównego Inspektora Sanitarnego z dnia 29 października 2012 r. w sprawie Programu Szczepień Ochronnych na rok 2013. Dziennik Urzędowy Ministra Zdrowia; Warszawa, dnia 30 października 2012 r.; poz. 78. Available from: http://dziennikmz.mz.gov.pl/DUM_MZ/2012/78/akt.pdf; [Accessed: 29 March 2013]
- Suárez E., Smith JS., Bosch FX. et al. Cost-effectiveness of vaccination against cervical cancer: a multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccine. 2008 Sep 15; 26 Suppl 5: F29-45
- Demarteau N., Detournay B., Tehard B., El Hasnaoui A., Standaert B. A generally applicable cost-effectiveness model for the evaluation of vaccines against cervical cancer. Int J Public Health. 2011 Apr; 56(2): 153-162
- Demarteau N., Tang CH., Chen HC., Chen CJ., Van Kriekinge G. Cost-effectiveness analysis of the bivalent compared with the quadrivalent human papillomavirus vaccines in Taiwan. Value Health. 2012 Jul-Aug; 15(5): 622-631
- Agencja Oceny Technologii Medycznych. Informacja w sprawie obowiązującej wysokości progu kosztu uzyskania dodatkowego roku życia skorygowanego o jakość. Available from: http://www.aotm.gov.pl/index.php?id=677; [Accessed: 11 April 2013]
- Charakterystyka Produktu Leczniczego Cervarix®. Available from: http://www.ema.europa.eu/docs/pl_PL/document_library/EPAR_-_Product_Information/human/000721/WC500024632.pdf; [Accessed: 25 March 2013]
- Kotarski J., Basta A., Dębski R. et al. Uzupełnione stanowisko Polskiego Towarzystwa Ginekologicznego dotyczące szczepień przeciwko zakażeniom wirusami brodawczaka ludzkiego (HPV) (stan wiedzy na dzień 19 września 2009 r.). Ginekol Pol 2009; 80: 870-876
- Chybicka A., Jackowska T., Dobrzańska A. et al. Zalecenia grupy ekspertów dotyczące pierwotnej profilaktyki raka szyjki macicy u dziewcząt i młodych kobiet. Pediatr Pol 2010; 85(4): 360-370
- Majewski S., Sikorski M. Rekomendacje Polskiego Towarzystwa Profilaktyki Zakażeń HPV (PTPZ-HPV) dotyczące stosowania profilaktycznych szczepionek przeciw HPV. Przew Lek 2008; 1: 222-227
- Majewski S., Sikorski M. Przełom w pierwotnej profilaktyce raka szyjki macicy i innych zmian związanych z zakażeniem HPV. Przew Lek 2007; 2: 108-113
- Badanie Zbigniewa Izdebskiego i Polpharmy Seksualność Polaków 2011. Available from: http://www.termedia.pl/badanie-zbigniewa-izdebskiego-i-polpharmy-seksualnosc-polakow-2011-,5152.html [Accessed: 18 July 2013]
- Lehtinen M., Paavonen J., Wheeler CM. et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012 Jan; 13(1): 89-99
- Paavonen J., Jenkins D., Bosch FX. et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007 Jun 30; 369(9580): 2161-2170
- Paavonen J., Naud P., Salmeron J. et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. The Lancet 2009 Jul 25; 374(9686): 301-314
- Szarewski A., Poppe WA., Skinner SR. et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15-25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer 2012 Jul 1; 131(1): 106-116
- Wheeler CM., Castellsague X., Garland SM. et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012 Jan; 13(1): 100-110
- WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. Available from: http://apps.who.int/hpvcentre/statistics/dynamic/ico/DataQuerySelect.cfm; [Accessed: 27 March 2013]
- Aubin F., Prétet JL., Jacquard AC. et al. Human papillomavirus genotype distribution in external acuminata condylomata: a Large French National Study (EDiTH IV). Clin Infect Dis. 2008 Sep 1; 47(5): 610-615
- Fahey MT., Irwig L., Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol 1995 APr 1; 141(7): 680-689
- Garland SM., Hernandez-Avila M., Wheeler CM. et al.Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007 May 10; 356(19): 1928-1943
- Harper DM., Franco EL., Wheeler CM. et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006 April 15; 367(9518): 1247-1255
- Insinga R., Glass A., Rush B. Health state transitions following an abnormal pap smear: implications for health utility assessment in cost-effectiveness analyses (abstrakt W-02 - 22nd International Papillomavirus Conference & Clinical Workshop 2005, Canada 2005)
- Munoz N., Kjaer SK., Sigurdsson K. et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010 Mar 3; 102(5): 325-339
- Myers ER., Green S., Lipkus I. Patient preferences for health states related to HPV infection: visual analog scale versus time trade-off elicitation (abstrakt nr 542 - Twenty-First International Papillomavirus Conference, México 2004)
- Smeets F., De Deken L., Baeten RFG. Cervixkankerscreening. Aanbeveling voor goede medische praktijkvoering. Huisarts Nu. 2002; 31(6): 275-295
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007 May 10; 356(19): 1915-1927
- Tjalma W., Paavonen J., Naud P. et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against abnormal cytology and low-grade histopathological lesions in an oncogenic HPV-naive population. Int J Gynecol Cancer 2009; 19(Suppl.2): 1008
- Woodhall SC., Jit M., Soldan K. et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect. 2011 Oct; 87(6): 458-463
- Medycyna Praktyczna. Indeks leków (dane z dnia 21 marca 2013 r.). Available from: http://indeks.mp.pl/; [Accessed: 21 March 2013]
- Armstrong EP.: Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types. J Manag Care Pharm. 2010 Apr; 16(3): 217-230
- Barnabas RV., Kulasingam SL. Economic evaluations of human papillomavirus vaccines. Expert Rev Pharmacoecon Outcomes Res. 2007 Jun; 7(3): 251-267
- Brisson M., Van de Velde N., De Wals P., Boily M.C. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007 Jul 20; 25(29): 5399-5408
- Colantonio L., Gómez JA., Demarteau N., Standaert B., Pichón-Rivière A., Augustovski F. Cost-effectiveness analysis of a cervical cancer vaccine in five Latin American countries. Vaccine. 2009 Sep 4; 27(40): 5519-5529
- Koleva D., De Compadri P., Padula A., Garattini L. Economic evaluation of human papilloma virus vaccination in the European Union: a critical review. Intern Emerg Med. 2011 Apr; 6(2): 163-174
- Marra F., Cloutier K., Oteng B., Marra C., Ogilvie G. Effectiveness and cost effectiveness of human papillomavirus vaccine. A systematic review. Pharmacoeconomics. 2009; 27(2): 127-147
- Nowakowski A., Jackowska T., Oszukowski PJ., Radowicki S., Wysocki J., Zatoński W. Profilaktyka raka szyjki macicy – problem interdyscyplinarny. Czy i jak możemy poprawić sytuację w Polsce?. Pediatr Pol. (2013). Available from: http://dx.doi.org/10.1016/j.pepo.2013.05.005; [Accessed: 10 October 2013]