Cost-effectiveness of injectable atypical long-acting antipsychotics for chronic schizophrenia in Poland
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Michiel E. H. Hemels |
Janssen Cilag, Birkerød, Denmark |
|
Thomas R. Einarson |
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON, Canada |
|
Roman Zilbershtein |
Pivina Consulting Inc., Mississauga, ON, Canada |
|
Agata Schubert |
Janssen, Warsaw, Poland |
|
Iwona Skrzekowska-Baran |
Janssen, Warsaw, Poland |
|
Kristel Van Impe |
Janssen, Neuss, Germany |
Objective: In order to determine the cost-effectiveness of paliperidone palmitate (PP-LAI), a long-acting injectable formulation, indicated for once-monthly injections as antipsychotic therapy, it was compared with risperidone, a long-acting, injectable (RLAI) and biweekly agent, administered for treatment of chronic schizophrenia in Poland, as perceived from the perspective of the National Health Fund (NHF).
Methods: We adapted a 1-year decision tree model to the Polish healthcare system with literature-derived and clinical expert inputs. The compared drugs included PP-LAI, a new treatment option of antipsychotic therapy, and RLAI, the established treatment for Polish patients. Clinical rates were derived from published trials. Model outputs included expected cost per patient, as well as the rates of hospitalization, emergency room visits, days free of symptoms and quality-adjusted life-years (QALYs). One-way sensitivity analyses were applied to major inputs. All the inputs were also simultaneously varied in probabilistic sensitivity analyses.
Results: Despite its higher acquisition cost, PP-LAI demonstrated a lower expected cost per treated patient. PP-LAI was associated with 0.824 QALYS, 323 days with stable disease and 44.6% hospitalization. RIS-LAI had 0.817 QALY, 317 stable days and 51.3% hospitalization. PP-LAI dominated RIS-LAI in the base case and in 55.0% of 10,000 simulations, and was cost-effective in 76.6%. However, the cost-effectiveness was sensitive, being lost with modest increases for PP-LAI or decreases for compared drugs with respect to their prices, relapse and adherence rates. Because it is injected monthly as opposed to biweekly, PP-LAI saves caregiver time as it is administered monthly, as opposed to the biweekly regimen.
Conclusions: From the viewpoint of the National Health Fund of Poland, when compared with RLAI, PP-LAI is a cost-effective drug with potential to reduce healthcare expenses.
Introduction
With a population of 38.4 million [1], Poland has got a healthcare system based on national health insurance, managed by the National Health Fund (NHF). NHF, with a total annual budget of €15 billion in 2012, allocates funds for hospital and outpatient care, as well as for prescribed, reimbursed drugs [2].
Schizophrenia is a major burden for healthcare systems, affecting about 1% of the world population [3]. The problem with schizophrenia is very serious and aggravating, due to intensive use of resources, including hospital beds and medications, where in-patient therapy represents a large cost centre [4,5].
Hospitalization costs are related to patients’ adherence to their antipsychotic medications [6]. Current innovative solutions, aimed at improving adherence rates, include the long-acting, injectable (LAI) depot formulations of drugs [7]. Although depots have been available for many years, atypical antipsychotic depots have been developed and marketed in the last decade only [8].
Even if these new products continue their upward trend on the market, their pharmacoeconomic profiles are a largely unknown issue in many countries, including Poland. A literature search was attempted to identify studies on the costs and economic aspects of schizophrenia therapies, which are available in Poland. Medline and Embase localised merely five relevant publications, all of them drafted as abstracts for poster presentations at scientific conferences [9-13]. No full text peer-reviewed articles were identified. Consequently, a task for defined and undertaken to determine the cost-effectiveness of atypical LAIs in Poland, the evaluation to be approached from the NHF’s analytic viewpoint.
Materials and Methods
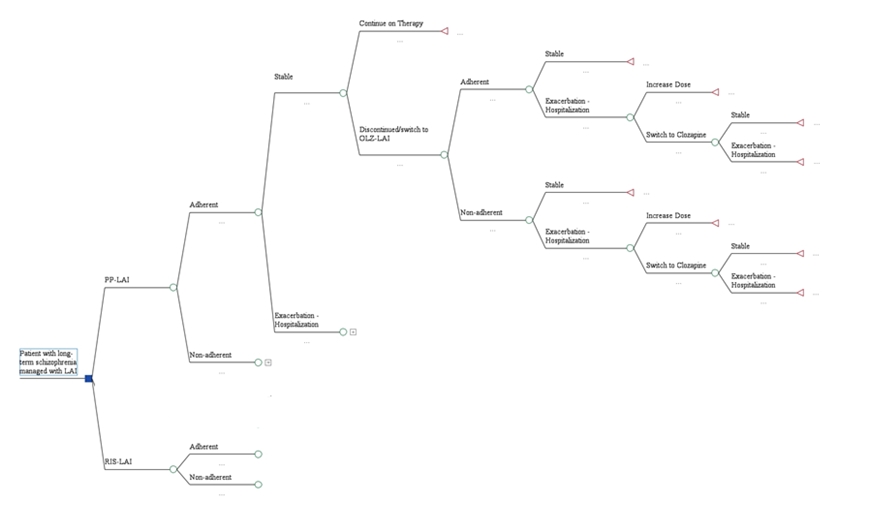
A model, previously developed in Greece [14], was adapted to the reality of the Polish healthcare system, see Figure 1. The introduced adaptations were based on published reports and inputs from local professionals.
Patients with chronic schizophrenia had experienced prior relapses. All of them required treatment with long-acting injectable (LAI) antipsychotics. They entered the model with their disease in remission. The drugs of interest included the long-acting injectable (LAI) forms of paliperidone palmitate (PP-LAI) and risperidone LAI (RLAI).
Clinical inputs were derived from clinical trials upon which success rates were based, or from daily clinical practice (i.e., medical records). Drug regimens and doses, used for maintenance therapy and to manage relapses, were based on published sources and adapted from the previous model (see Table 1).
Table 1. Regimens used to treat patients with schizophrenia, with appropriate references
| Drug | Reason for administration | Regimen | Source | |||
| PP-LAI | Maintenance of stable schizophrenia | 69.3 mg monthly | Average of Fleischhacker [31], Gopal [32] |
|||
| Treatment of relapse | 150 mg week 1, 100 mg week 2; then 84.9 mg every 4 weeks maintenance | EMA Xeplion® product summary [33]; Maintenance dose = average of Gopal[34], Hough[35], Nasrallah [36], Pandina [37], Pandina [38] | ||||
| RIS-LAI | Maintenance of stable schizophrenia | 40.3 mg every 2 weeks | Average of Fleischhacker [39], Kissling[40], Lasser[41], Lee[42], Olivares[43] | |||
| Treatment of relapse | 50 mg every 2 weeks | Average acute dose set to the maximum of 50 mg, pro-rated from PP-LAI ratio of acute:maintenance doses and validated by Chue[44], Eerdekens[45], Kane[46] | ||||
| EMA, European Medicines Agency; LAI, long-acting injection; PP, paliperidone palmitate; RIS, risperidone microspheres. |
||||||
The clinical rates, used to populate the decision tree, are presented in Table 2. The analysis was arranged from the point of view of the NHF and considered direct costs of care only. The direct costs included medications, hospitalization and medical care (i.e., services provided by physicians and other healthcare professionals). Drug prices were obtained from the current price list, published by the Ministry of Health [15]. The service prices were based on the NHF procedure pricing system and on opinions of professionals. The costs were provided in EURO for the 2012 exchange rate, adjusted from other years by the Consumer Price Index for Poland [16]. The cost inputs are exhibited in Table 3.
Table 2. Clinical rates used as inputs for the model
| Clinical state | Drug | Adherent | Non-adherent |
| Adherence | PP-LAI | 0.872 | 0.118 |
| RIS-LAI | 0.823 | 0.177 | |
| Stable disease | PP-LAI | 0.803 | 0.148 |
| RIS-LAI | 0.763 | 0.14 | |
| Relapsed | PP-LAI | 0.197 | 0.852 |
| RIS-LAI | 0.237 | 0.86 | |
| *Rates adopted from previous model | |||
Table 3. Consumed resources and their costs
| Resource | Item | Cost (Euro) |
| Hospital | Acute psychiatric ward | 42.06 |
| Emergency room (2 hours) | 26.72 | |
| Long term ward | 28.04 | |
| Medical | Psychiatric outpatient visit | 13.17 |
| Follow-up visits | 6.58 | |
| Primary care physician (hour) | 4.66 | |
| Allied healthcare | Psychiatric nurse (hour) | 4.66 |
| Drugs | PP-LAI* | €9.30/day |
| RIS-LAI | €8.46/day | |
| *Estimated costs as not yet reimbursed | ||
The primary outcome was quality adjusted life years (QALYs). Their calculation was based on previously obtained utility scores, reported in the literature [17-20]. Other patient outcomes included days free of symptoms and the rates of relapse. The expected cost per treated patient was calculated for each drug. The incremental cost-effectiveness ratio (ICER) for gained QALYs was interpreted as economic outcome. Ratios below €25,000 were considered cost-effective, as per PolAHTA guidelines [21].
In order to examine the model stability and obtainable results, a series of sensitivity analyses was run. Each of the major inputs (e.g., adherence rates, hospitalization rates, costs, etc.) was tested with one-way (break-even) analyses to determine if obtained results would change within reasonable limits. Also, a probabilistic (Monte Carlo) synthesis was undertaken with 10,000 RLAI comparing iterations. The rates and costs at each decision node varied across a plausible range, using standard distributions for each variable. The proportions of iterations were also calculated, which favoured PP-LAI and comparison drugs. Two sets of outcomes were examined. In the first one, the threshold values were explored for dominance; in the second one, the threshold for cost-effectiveness was analysed, using the limits, as established by PolAHTA.
Results
Even with higher acquisition cost, PP-LAI would have a lower expected cost per treated patient, when the benefits are included in the estimation model (Table 4). PP-LAI was associated with 0.824 QALYS, 323 days with stable disease and 44.6% hospitalization. RIS-LAI had 0.817 QALY, 317 stable days and 51.3% hospitalization. PP-LAI dominated RIS-LAI in the base case and in 55.0% of 10,000 simulations, and was cost-effective in 76.6%. However, the cost-effectiveness was sensitive and lost with even modest increases for PP-LAI or with decreases for compared drugs with respect to drug prices, relapse and adherence rates. Because it is injected monthly as opposed to biweekly, It also saves caregiver’s time, being injected monthly, as opposed to biweekly regimens.
Table 4 Clinical and pharmacoeconomic outcomes
| Drug | Expected cost/ patient (EURO) | Remission days | Hospitalization rate | QALYs/ patient | Cost (EURO)/ QALY | Economic outcome |
| PP-LAI | 3604 | 323.4 | 44.60% | 0.824 | 4373 | dominant |
| RIS-LAI | 3648 | 317.3 | 51.30% | 0.817 | 4467 | dominated |
Discussion
Despite its higher acquisition cost, PP-LAI demonstrated the lowest expected cost per treated patient. Because its therapeutic effect lasts a full month, having a reasonable side effect profile [22], its adherence rates are fairly high. Consequently, it exhibits higher efficacy, since adherence has been identified as the major driver of costs and patient status [23,24].
These results suggest that PP-LAI should be the atypical LAI of choice in Poland. Since more patients remain in stable condition, a broader adoption of the therapy should result in fewer hospital admissions, reducing patient loads on hospitals.
No indirect costs were taken into account in this analysis. The impact of the therapy on the number of sick leave episodes was assumed to be minimal, considering the very low employment level of schizophrenic patients; however, it is also possible that this medication could allow some of the patients to resume work or, at least, function more efficiently at home [25,26]. Other disregarded indirect costs included those, associated with the legal and justice system. It is well known that a proportion of persons with schizophrenia become violent and are frequently incarcerated, often many times [27,28]. Finally, no costs of adverse events were incorporated. At least two government agencies concluded that side effects contributed very little to the overall treatment costs [29,30]. Therefore, the results represent a conservative estimate with some underestimation of the total cost. Nonetheless, all of these aforementioned biases would be against PP-LAI.
Limitations
All research reveals certain limitations, either due to selected approach or the conduct of research tasks or for any other reason. A decision tree model was employed to simulate treatment and its outcomes. The results are therefore limited by the validity of inputs and the assumptions made in the modelling process. Decision trees estimate the average cost for the average patient under average conditions. The results apply therefore only to patients who meet inclusion criteria. They may or may not apply to related diseases, such as schizoaffective, schizophreniform, or bipolar disorder. The model was also limited to patients with chronic schizophrenia without comorbid conditions. Any extrapolations to other populations should thus be done with caution.
Inputs, specific for Poland, were used in this analysis, being, however, limited by the unavailability of certain data. In cases where some information was not available, we used data from similar environments in other countries or from multi-country trials, which may or may not have included patients from Poland. Local experts were also enquired to counsel concepts, validate assumptions and assure that inputs were appropriate and applicable.
In calculations, no co-payments were considered, assuming that they would be similar across drugs and would therefore not affect the outcomes to any great extent. The impact of that assumption is, however, not known.
Conclusions
In this model, PP-LAI dominated the other atypical LAIs. Therefore, it is perceived as an atypical LAI of choice for patients with chronic schizophrenia. From the viewpoint of the National Health Fund of Poland, PP-LAI is a cost-effective drug with real potential to reduce healthcare expenses.
- Poland Demographics Profile 2012: Population. Available from: http://www.stat.gov.pl/gus; Central Statistical Office; [Accessed: 2013-10-03]
- NHS Plan for report 2012. Available from: http://www.nfz.gov.pl/new/index.php; [Accessed: 2013-10-03]
- Tandon R., Keshavan MS., Nasrallah HA. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophr Res 2008; 102: 1-18
- Rössler W., Salize HJ., van Os J., Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 2005; 15: 399-409
- Knapp M., Mangalore R., Simon J. The global costs of schizophrenia. Schizophr Bull 2004; 30: 279-93
- Weiden PJ., Kozma C., Grogg A., Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004; 55: 886-91
- Nasrallah H. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand 2007; 115:260-7. Am J Psychiatry 2003; 160: 1125-32
- Kane J., Eerdekens M., Lindenmayer J-P., Keith S., Lesem M., Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003; 160: 1125-32
- Kostrzewska K., Lis J., Glasek M., Rys P., Wladysiuk M., Plisko R. Cost-utility of amisulpride compared with first generation antipsychotics in treatment of schizophrenia in Poland. Value Health 2011; 14: A292
- Faluta T., Rdzanek M., Pierzgalska K., Wrobel B. The economic and social consequences of schizophrenia treatment with seroquel XR (quetiapine prolonged release tablets) in Poland: Analysis of the impact on the health care system. Value Health 2-11; 14: A289-A290
- Walczak J., Stelmachowski J., Obrzut G., Nogas G. Cost-utility analysis (CUA) of sertindole in the treatment of schizophrenia in Poland in general population of schizophrenic patients and population of patients intolerant to at least one other antipsychotic agent: A five-year markov model. Value Health 2010; 13: A453
- Walczak J., Augustynska J., Soltys E., Nogas G. Budget impact analysis of sertindole in the treatrment of schizophrenia in Poland. Value Health 2010; 13: A446-A447
- Wilk D., Rutkowski J., Dziewiatka M., Lis J., Glasek M., Plisko R. Budget impact analysis of amisulpride in treatment of schizophrenia in Poland. Value Health 2010; 13: A447
- Einarson TR., Geitona M., Chaidemenos A. et al. Pharmacoeconomic analysis of paliperidone palmitate for treating schizophrenia in Greece. Ann Gen Psychiatry 2012 Jul 2; 11(1): 18
- Reimbursement list. Available from: http://www.mz.gov.pl/wwwfiles/ma_struktura/docs/obwieszczenie_4_26102012.pdf; [Accessed: 2013-10-03]
- Poland Inflation rate (consumer prices). Available from: http://www.indexmundi.com/poland/inflation_rate_(consumer_prices).html; [Accessed: 2012-11-20]
- Briggs A., Wild D., Lees M. et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes 2008; 6:105
- Cummins C., Stevens A., Kisely S. The use of olanzapine as a first and second choice treatment in schizophrenia. Birmigham, UK: Department of Public Health & Epidemiology, University of Birmingham. A West Midlands Development and Evaluation Committee Report; 1998
- Lenert L., Sturley A., Rapaport M. et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schiz Res 2004; 71: 155-65
- Oh P., Lanctôt K., Mittmann N. et al. Cost-utility of risperidone compared with standard conventional antipsychotics in chronic schizophrenia. J Med Econ 2001; 4: 137-56
- Cost-effectiveness threshold information. Availablefrom: http://www.aotm.gov.pl/index.php?id=677; [Accessed: 2013-10-04]
- European Medicines Agency. Xeplion® summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002105/WC500103317.pdf; [Accessed: 2012-11-20]
- Svarstad BL., Shireman TI., Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv 2001; 52: 805-11
- Ascher-Svanum H., Faries DE., Zhu B., Ernst FR., Swartz MS., Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry 2006; 67: 453-60
- Rymaszewska J., Mazurek J. The social and occupational functioning of outpatients from mental health services. Adv Clin Exp Med 2012; 21: 215-23
- Rymaszewska J., Jarosz-Nowak J., Kiejna A. et al. Social disability in different mental disorders. Eur Psychiatry 2007; 22: 160-6
- Vevera J., Hubbard A., Veselý A., Papezová H. Violent behaviour in schizophrenia. Retrospective study of four independent samples from Prague, 1949 to 2000. Br J Psychiatry 2005; 187: 426-30
- Bo S., Abu-Akel A., Kongerslev M., Haahr UH., Simonsen E. Risk factors for violence among patients with schizophrenia. Clin Psychol Rev 2011; 31: 711-26
- All Wales Medicines Strategy Group. Final appraisal report: Olanzapine depot (ZypAdhera®), Lilly UK. Advice No: 1510 – October 2010. Available from: http://www.wales.nhs.uk/sites3/Documents/371/olanzapine%20depot%20(ZypAdhera)%20schizophrenia.pdf; [Accessed: 2012-11-20]
- Farahati F., Boucher M., Williams R., Williams R., Herrmann N., Silverman M. et al. Atypical antipsychotic monotherapy for schizophrenia: clinical review and economic evaluation of first year of treatment [Technology report number 91]. Ottawa: Canadian Agency for Drugs and Technologies in Heath 2007
- Fleischhacker WW., Gopal S., Lane R. et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neurospychopharmacol 2011; Jul: 1-12
- Gopal S., Vijapurkar U., Lim P., Morozova M., Eerdekens M., Hough D. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 2010; 25: 685-97
- European Medicines Agency. Xeplion® summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002105/WC500103317.pdf; [Accessed: 2012-11-20]
- Gopal S., Hough D., Xu H., et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol 2010; 25: 247-56
- Hough D., Lindenmayer J-P., Gopal S. et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1022-31
- Nasrallah HA., Gopal S., Gassmann-Mayer C. et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 2010; 35: 2072-82
- Pandina GJ., Lindenmayer J-P., Lull JM. et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychiatry 2010; 30: 235-44
- Pandina GJ., Lane R., Gopal S. et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 218-26
- Fleischhacker W., Eerdekens M., Karcher K. et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003; 64: 1250-7
- Kissling W., Heres S., Lloyd K. et al. Direct transition to long-acting risperidone - analysis of long-term efficacy. J Psychopharmacol 2005; 19: 15-21
- Lasser R., Bossie C., Gharabawi G., Baldessarini R. Clinical improvement in 336 stable chronically psychotic patients changed from oral to long-acting risperidone: a 12-month open trial. Int J Neuropsychopharmacol 2005; 8: 427-38
- Lee M., Ko Y., Lee S. et al. Long-term treatment with long-acting risperidone in Korean patients with schizophrenia. Hum Psychopharmacol 2006; 21: 399-407
- Olivares J., Rodrigues-Morales A., Diels J. et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: Results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry 2009; 24: 287-96
- Chue P., Eerdekens M., Augustyns I. et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005; 15: 111-7
- Eerdekens M., Van Hove I., Remmerie B., Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004; 70: 91-100
- Kane J., Eerdekens M., Lindenmayer J-P., Keith S., Lesem M., Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003; 160: 1125-32