Medical Information Center (CIM)
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Wojciech Giermaziak |
- |
Medical Information Center (CIM)
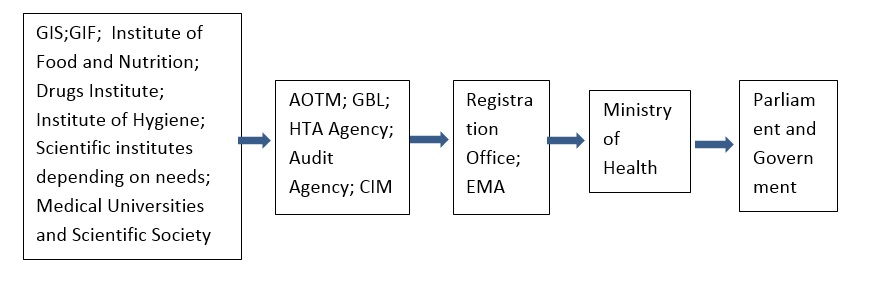
Many institutions deal, or should deal with the collection, processing and purposeful use of the available scientific information in the world, which targeted, will become an instrument in the activities of the government. These institutions are acting separately, often using information from unverifiable sources, creating imperfect opinions, potentially used by the bureaucratic apparatus. Amplification of that imperfection in the form of an administrative decision is resulting as quality of established law and consequently efficiency of the state, and the quality of its image in the world.
The proposal to create the Medical Information Centre can provide a solution that entering similar, already existing European and national structures, with similar, usually narrowly specialized databases of information or opinion-forming units become the most serious, independent of the influence of lobbying factors tool for the state in the making and amendment of systems and procedures valid in the Polish health care.
The above diagram of the organizational and functional Medical Information Center assumes the use of the potentials of four units:
-
GBL - its scientific background in the form of on-line Thesaurus, Polish Medical Bibliography, international databases,
-
AOTM - in accordance with its statutory purpose, in my opinion should not be used only for the purposes of public payer, but for the assessment of all procedures required evaluation of the proper functioning of the health care system in Poland,
-
Medical Audit Agency - control the efficiency of the system,
-
HTA - the state agency, which guarantees the quality of medical records evaluation serving as the starting material for the registration work and authorization of drugs, medical supplies and medical devices for URPL.
The sources of current information on all aspects of the Polish market of drugs, from registration procedures, to control of the market and the internal control procedures, are:
-
Chief Sanitary Inspectorate (GIS) - should fulfill functions in accordance with statutory appointment. Thus, I want to clearly emphasize that this is not the authority of the State entitled to use the procedures for registration and admission to trading drugs without a prescription and dietary supplements. This feature fulfill URPL appointed by relevant law , therefore any existing GIS competence on drugs and dietary supplements should be immediately transferred to the URPL - proper authority of law, after a proper assessment of the merits and qualifications in the Medical Information Center . GIS should analyze and control the national and international market as specified in the Act.
-
Main Pharmaceutical Inspectorate (GIF) - similarly as GIS in addition to control of drugs and medical products manufacture should after the amendment or writing a new Pharmaceutical Law play the role of the pharmaceutical police, controlling obeying of the law in companies operating in the Polish pharmaceutical market in cooperation with the police, border guards, the Polish army, fiscal institutions and, if necessary and justified with special services. The security interests of the state on the internal market with the principles of the protection of the Polish companies interests (including state protectionism in selected directions of development of the Polish market of drugs from production, import, export to pharmacy retail). The basis for the effective working of the GIF as in the case of GIS is transfer of decision-making in the hands of the government which is the guarantor of efficiency.
-
Chief Veterinary Inspectorate - I have not included the role of the Inspectorate in the sources for the CIM, but I think that in terms of drug production and marketing under the supervision of the Ministry of Agriculture with supervision limited to issue permits for veterinary wholesalers by GIF and supervision of production, requires law changes, specially that many "human" drugs are used on a daily basis in veterinary medicine. In my opinion, the market in this area is beyond the control of the State. The quality of animal and crop production as a raw food has the impact on level of public health and spendings on the remedy.
-
Medical Universities in Poland and cooperating with them (through them) research centers and institutions of other countries, as well as it does today in the GBL, (which is the general distributor of WHO scientific information on Poland) international organizations.
Similarly, Institutes and Research Societies. The purpose of such solutions is the government access to the latest of Polish and world scientific achievements professionally prepared and thereby providing a source to develop the most optimal system solutions for the Polish health service. World adopted system solutions, after checking their social function and actions quality of already implemented examples, are proven method of adaptation of the best methods to deal with simultaneous elimination of lower quality solutions.
Obtained information from all signaled above sources, provide the basis for improving the functioning of the registration and admission system of trading dietary supplements and medical products on the Polish market in a way that ensures the safety of their use.
Improvement of quality and transparency in this economy area generates by itself significant savings in the system and the accuracy of management so saved up resources in the most optimal way in terms of function and application of Union and national law. For example the action of the Economic Commission at the Ministry of Health or the previous, however, strongly narrowed actions of AOTM in assessing drug technologies.
Another positive factor affecting the quality of the system and in favor of the CIM acceptance as a system solution is a substantive justification for the revision or creation of a new medical law, by identifying the most secure in the assessment of international researches, solutions that should be implemented into national law.
Additional positives resulting from the implementation of the systemically uniform, existing today as separate entities organization - the authorities of the state are:
-
the quality of medical information,
-
reliability of the information,
-
the acquisition speed of decision-making authorities,
-
independence from the lobbying influence for the opinion creation,
-
cooperation with other government units as component of the stabilizing role of the state in the organization and management of the health care system,
-
with rational and controlled risk management, CIM establishment does not carry additional state expenditures beyond the already existing for separate units included in the CIM,
-
cooperation with CIM analogues in the EU and in the world in the direction of optimizing the Polish health care system as part of integrated systems in Europe, and in some aspects (eg. vaccines and vaccination) in the world.
The above system changes previously existing, not always effective and efficient, circulation medical information system in Poland, perhaps in some circles it is going to be seen as too revolutionary, but I think that insertion of it, as the solution adopted in many EU countries (eg Italy, Spain, the Netherlands, Norway ) can be an innovative step in the quality of substantive administrative decision in Polish drug market, and the strengthening of state control in the most socially vulnerable area such as the public health. I am aware of the need to discuss the details of this proposal, but it seems that such a discussion soon can lead to a radical improvement in the health sector by providing substantive answers to the most difficult questions and issues that should be resolved as soon as possible. I hereby declare my participation in substantive discussions on the proposed project and collaboration at every stage of its implementation.