The overview of Agency for Health Technology Assessment recommendations in 2012, and their impact on reimbursement decisions
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Patrycja Prząda-Machno |
Business School, Warsaw University of Technology, Warsaw, Poland |
|
Marcin Czech |
Business School, Warsaw University of Technology, Warsaw, Poland |
An introduction of the Reimbursement Act in Poland, which took effect on 1st January 2012, was one of the most important reforms implemented in the Polish healthcare system over the past few years, affecting significantly the pharmaceutical market and its stakeholders.
Objective: The aim of this study was to analyse the market access of new, innovative therapies for the Polish patients, after the Reimbursement Act being in force for over one year.
Methods: The analysis was based on the Overview of the Minister of Health Orders, published on the Polish Agency for Health Technology Assessment (AHTAPol) website. The AHTAPol President and Council for Transparency’s recommendations were divided into the positive and negative ones, and they were further divided into their corresponding reimbursement categories: the register of reimbursed medicines, drug programs and the chemotherapy catalogue. The issued recommendations were than compared with the final reimbursement decisions made by the Minister of Health.
Results: In the analysed period AHTAPol issued 46 positive / positive subject to conditions recommendations of which: 31 (69%) concerned the medicines available in drug programs, 10 (22%) concerned the medicines available on prescription in pharmacy, and 4 (9%) concerned the products available in chemotherapy catalogue. Most medicines that had been positively recommended were reimbursed from public funds and made available for the Polish patients.
Conclusions: Most positively recommended by AHTAPol technologies received positive reimbursement decisions. Receiving a negative recommendation did not imply the negative reimbursement decision.
Introduction
The introduction of the 12th May 2011 Reimbursement of Medicinal Products, Food Products for Particular Nutritional Purposes, and Medical Devices Act (Polish: Dz.U. 2011 nr 122 poz. 696), hereinafter referred to as the Reimbursement Act, which took effect on 1st January 2012, was one of the most important reforms implemented in the Polish healthcare system over the past few years. The Reimbursement Act significantly affected the medicine market parties, including: the payer, providers, beneficiaries, pharmaceutical companies, pharmaceutical wholesalers and pharmacies.
The introduction of Reimbursement Act entailed, among others, that the provisions of EU Transparency Directive are implemented in the Polish reimbursement laws, and the Polish reimbursement laws are adjusted as to correspond to the EU requirements. The objective of Reimbursement Act was both to ensure more transparency in pricing mechanisms and medicinal products reimbursement in Poland, and to rationalize the National Health Fund expenditure. The decision-makers announced greater control over the NHF budget, which would result in creating new funding opportunities for innovative products to be offered to the Polish patients.
As a result of the alterations introduced in 2012, the reimbursement application and assessment procedures have also changed. As stated in the Reimbursement Act, pharmaceutical company is the only entity eligible to apply for product reimbursement, as well as for the increase, decrease or any revision of its official selling price. Furthermore, pharmaceutical company may request to shorten the reimbursement decision expiry period. Applications for product reimbursement and for official selling price increase are one of the most extensive ones, both for the applying party and the institutions assessing them (such as the Ministry of Health and the Agency for Health Technology Assessment). Whether the applications are submitted for original drugs or the generic ones, they contain a set of the HTA analyses, which allows for the assessment of clinical benefits resulting from the use of a drug, as well as financial implications of such reimbursement, both for the payer and the patient [2].
The Reimbursement Act defines precisely the reimbursement application scope, its assessment period, as well as each party’s, i.e. the Ministry of Health or the Agency for Health Technology Assessment, contribution to its verification.
Pharmaceutical companies seeking reimbursement are required to submit pricing and reimbursement application to the Ministry of Health containing the following information:
- the description of the subject of the application;
- proof of the availability of the drug on the market at the time of the submission of the application;
- the undertaking to ensure continuity of supply, together with an indication of the annual volume of supplies in event of inclusion in the reimbursement;
- data identifying the drug (the name, form, method of administration, type of packaging;
- the authorisation number and a copy of the marketing authorisation decision,
- the EAN ID code or other code corresponding to the EAN code;
- the requested conditions for inclusion in the reimbursement, in particular the indications for which the drug is to be reimbursed; the proposed net sales price; the category of reimbursement availability, the level of payment; the risk-sharing instruments, the period of inclusion in the reimbursement; the draft description of the regimen programme (if applicable);
- the maximum and minimum net sales price of the drug in Poland during the year before the submission of the application;
- the maximum and minimum net sales price during the year before the submission of the application in the EU and EFTA countries in which the drug is reimbursed;
- the daily cost of therapy, average cost of standard therapy, duration of standard therapy separately for each indication;
- information on the expiry of patent protection, including the additional protection certificate and also the expiry of data exclusivity and market exclusivity period [1].
Pharmaceutical companies are obliged to deliver in reimbursement application a justification of the application containing budget impact analysis (BIA) detailing the overall cost to the National Health Found of reimbursing the drug [1].
For a drug which has no reimbursement counterpart in the given indication the following information is also required:
- a clinical analysis, prepared on the basis of a systematic review compared with other medical procedures which can possibly be used in the given clinical condition with respect to the indication for which the application was submitted;
- an economic analysis from the point of view of the entity obliged to finance the drug;
- analysis of the impact on the budget of the entity responsible for financing the drug;
- rationalisation analysis, presented if the analysis of the impact on the budget of the entity obliged to finance drugs with public funds indicates an increase in the cost of reimbursement; this analysis should provide detailing reimbursement solutions to free up public funds [1].
It should be noted that all analyses must be up-to-date as of the date of application submission, in the scope of efficacy, safety, prices as well as the level and method of financing [1].
The Ministry of Health Minimal Requirements for HTA & the AHTAPol Guidelines in details describe requirements for HTA dossier framework.
According to article 6 Reimbursement Act, drugs are available in specific category of reimbursement. Pharmaceutical companies can apply for reimbursement in the specific category of reimbursement which is the appropriate approach for their drug.
We can distinguish four types of reimbursement:
- drug available in the pharmacy on prescription- the drug is added to the list of reimbursed drugs
- drugs used in a regimen program- kind of reimbursement for high-cost innovative drugs,
- drug used in chemotherapy;
- drug used within the framework of the provision of guaranteed benefits, other than those listed above [1].
In case of the applications submitted with a set of HTA analyses, it is the AHTAPol who is responsible for their assessment. As the advisory body for the Ministry of Health, the AHTAPol is to verify the submitted HTA reports, and issue recommendations for each health technology funding, based on whether the application was positively verified. Agency for Health Technology Assessment operates in accord with the tasks assigned to it by the Minister of Health. In accord with Article 31 c of the 27th August 2004 Act on the Healthcare Provisions and Services Financed from Public Funds (Polish: Dz.U. 2008Nr 164 poz. 1027 ze zm.), the Minister of Health obliges the AHTAPol President to issue recommendations, statements or opinions assessing healthcare provisions and services.
The aim of study
The aim of this study is to analyze the innovative therapies availability for the Polish patients, after the Reimbursement Act being in force for over one year.
Methodology
This analysis was made based on the Overview of Minister of Health Orders, published on the Agency for Health Technology Assessment website.
In 2012, the Agency for Health Technology Assessment (hereinafter AHTAPol) received 132 orders from the Minister of Health. 13 of them were suspended or withdrawn by the Minister of Health, therefore AHTAPol processed 119 orders demanded by the Minister of Health.
Most of these orders, i.e. 68, concerned reimbursement applications (out of this number, 5 applications were suspended). Therefore, there were 63 recommendations analyzed in this work. The AHTAPol President and the Transparency Council recommendations were then divided into the positive and negative ones, and they were further divided into their corresponding reimbursement categories: the list of reimbursed drugs, drug programs, the chemotherapy catalog. The final part of this analysis was to assess and evaluate the issued recommendations, and relate these to the Minister of Health reimbursement decision, based on the 26th August 2013 Minister of Health Proclamation of the Register of Reimbursed Medicines, Food Products for Particular Nutritional Purposes, and Medical Devices, which came to force on 1st September 2013.
Results
The AHTAPol is responsible for assessing the reimbursement applications submitted by pharmaceuticals companies of new drugs, as well as price increase applications for existing reimbursed drugs.
AHTAPol assessment is conducted in three phases:
- initial assessment which result is the verification analysis of the clinical and economic data submitted with the reimbursement application. This part of the assessment is to ensure that the analysis were prepare in accordance to law and guidelines;
- opinion of the Transparency Council for the President of the AHTAPol Transparency Council is the independent body within the AHTAPol made up of medical experts, representatives of the Ministry of Health and the National Health Found who’s are responsible for ensure clear and transparent process of the assessment and prepare the opinion for President of AHTAPol
- recommendation of the President of the AHTAPol prepare for the Minister of Health taking in to account the opinion of the Transparency Council (at a later stage of the process is used by the Economic Committee as part of the price negotiation with pharmaceuticals companies.).
As previously mentioned in 2012 AHTAPol assessed 63 orders which concerned reimbursement applications. Orders were related to 61 drugs, in the case of 2 products orders differed only EAN code.
Positive/ conditional positive recommendation
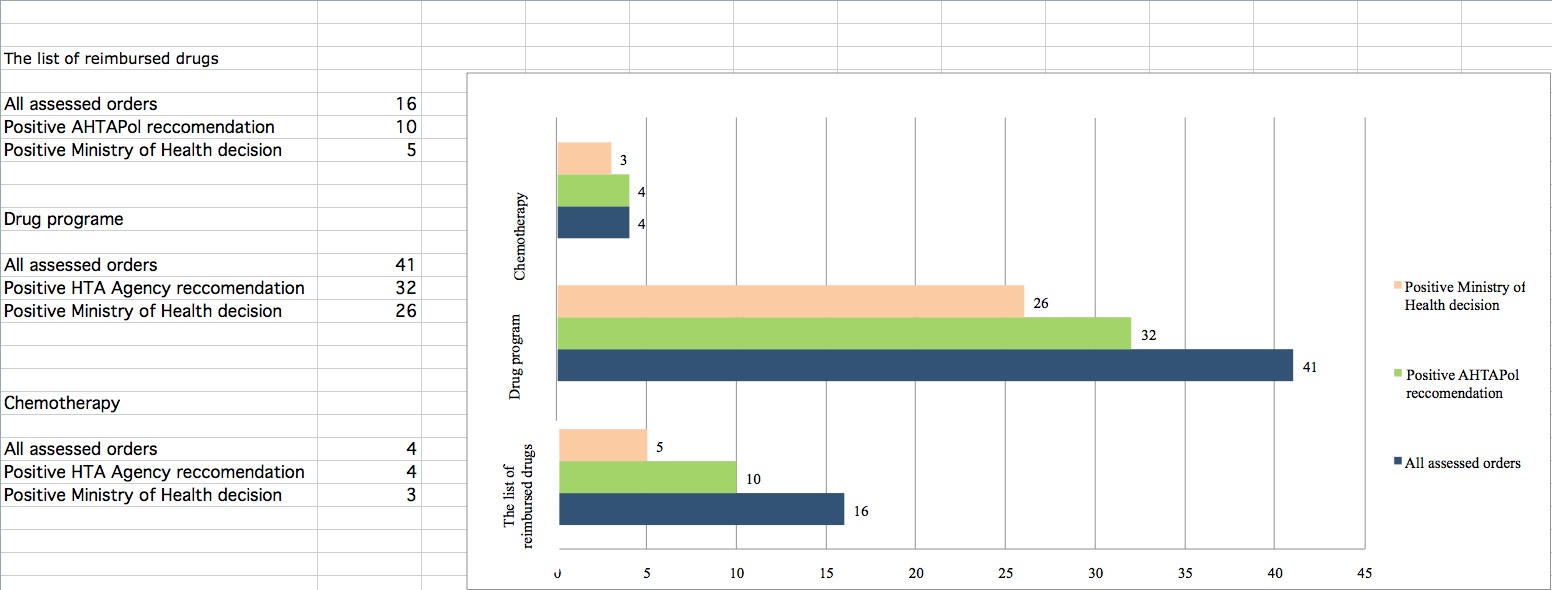
Based on the analyses conducted in this study, we can conclude that in 2012, AHTAPol issued 46 positive / conditional positive recommendations (Figure 1), of which:
32 (69%) concerned the medicines available in drug programs,
10 (22%) concerned the medicines available on prescription in pharmacy,
4 (9%) concerned the products available in chemotherapy catalog.
Out of 32 positive recommendations issued for the medicines to be reimbursed within drug programs, 18 were not conditioned in any way. After analyzing the remaining 14 recommendations, it was concluded that the major factor for them being granted conditioned recommendations was that the costs of therapy were too high, which translated into a medicine failing to achieve the acceptable cost-effectiveness. In most cases, the AHTAPol President recommended either extending or proposing a more suitable risk sharing schemes, as to achieve the acceptable cost-effectiveness. In almost all these cases, the Transparency Council statements were consistent with the AHTAPol President recommendations. It was only in case of conestat alfa that there was discrepancy noted. The AHTAPol President recommended this product, based on its evidenced effectiveness and its safety for use. In their opinion, conestat alfa seemed a cheaper and safer alternative to the product now in-use. On the contrary, in their statement, the Transparency Council decided it is unfounded to reimburse this medicine within the drug program, and suggested it is reimbursed subject to conditions within the register of reimbursed medicines [4].
There were 10 positive recommendations issued for the medicines available on prescription in pharmacies. Most of these positive recommendations (6 out of 10) were not subject to any condition for the liable party to meet. In 4 cases, it was noted in the course of this study, that the AHTAPol President did not accept the proposed risk sharing tool (e.g. in case of ivabradine, or recommended to introduce a more suitable one (e.g. (Nutramigen LGG; letrozole). Furthermore, in case of Nutramigen LGG, the AHTAPol President specified the population for which the medicine can be used, and suggested a different apportionment (payment level) than it was proposed by the applicant [5,6].
There were 4 positive recommendations issued for the medicines available in chemotherapy catalog. It was only in one case that the AHTAPol Presidents conditioned their decision on lowering the price of thalidomide to the price of 1 mg of thalidomide, a substance currently financed within the target import. Furthermore, in the course of clofarabine assessment, there was noted discrepancy in the Transparency Council and the AHTAPol President opinions. In their statement, the Transparency Council, declared it is unfounded to reimburse this medicine within the Register of Active Substances Used in Cancer Chemotherapy, while they nonetheless recommended the medicine to be reimbursed within the drug program. The Council also suggested the medicine producer to lower its price as to achieve the acceptable cost effectiveness. The AHTAPol President, on the contrary, did not condition their positive recommendation in any way, and suggested the medicine to be reimbursed in the same reimbursement availability category as proposed by the applicant [7,8].
Negative recommendation
The analysis of 64 orders showed that AHTAPol issued 15 negative recommendations (Figure 2), of which:
9 (60%) concerned drugs available in drug programs,
6 (40%) concerned drugs available on prescription in pharmacies
Within one of the reimbursement availability categories in Poland, namely drug programs, the AHTAPol President issued 9 negative recommendations. In almost all these cases, the major factor for issuing a negative recommendation was that the clinical analysis was found insufficiently reliable. The AHTAPol President contested the reliability of research results to be assessed in terms of effectiveness and safety for use, hence, the medicine effectiveness and safety were found insufficient. In 5 out 9 cases, the assessed medicines were found cost-ineffective in relation to the limit set in the Reimbursement Act as the limit of cost effectiveness of medicines in Poland. In 2 cases, despite their negative recommendation, the AHTAPol President highlighted it is worthwhile to reimburse eltrombopag and alglucosidase alfa subject to three conditions: that there is a risk division tool introduced, that the drug program is properly monitored, and that the solutions proposed by the applicant are made part of the rationalization analysis [9,10]. The analysis of 6 negative recommendations issued for the products to be reimbursed within the open register showed that in each case, the medicine effectiveness was similar to the effectiveness of medicines already in use and reimbursed within the same register. Hence, there was no evidence that their technology was superior to the technology of medicines already reimbursed from public funds. Furthermore, in two cases (i.e. denosumab, etrabenazine), the applicant did not demonstrate sufficient cost-effectiveness as required by the cost-effectiveness limit mandatory in Poland[11,12]. The analysis of negative recommendations issued for the following products: Lercanidipini hydrochloridum, rotigotine, levetiracetam) showed also that these medicines would generate higher treatment costs than the medicines already reimbursed [13,14,15,16].
Ministry of Health decision
Based on the 26th August 2013 Minister of Health Proclamation of the Register of Reimbursed Medicines, Food Products for Particular Nutritional Purposes, and Medical Devices, which came to force on 1st September 2013, in this study, there were positive and negative recommendations issued by the AHTAPol President, analyzed, along with their impact on final reimbursement decisions.
In Accordance to the Reimbursement Act Minister of Health makes reimbursement decision based on the: position of the Economic Commission, the recommendation of the President of the Agency; the clinical effectiveness and safety of the drug; the relationship between the risks and benefits associated with the treatment; cost ( e.g. versus existing alternative therapies), price competitiveness; the impact on the expenditures of the public payer and patients; public healthcare priorities and finally the level of the cost-effectiveness threshold which is defined as cost of achieving an additional year of life adjusted by quality (QALY) and it is set at an amount of three times the Gross Domestic Product per capita.
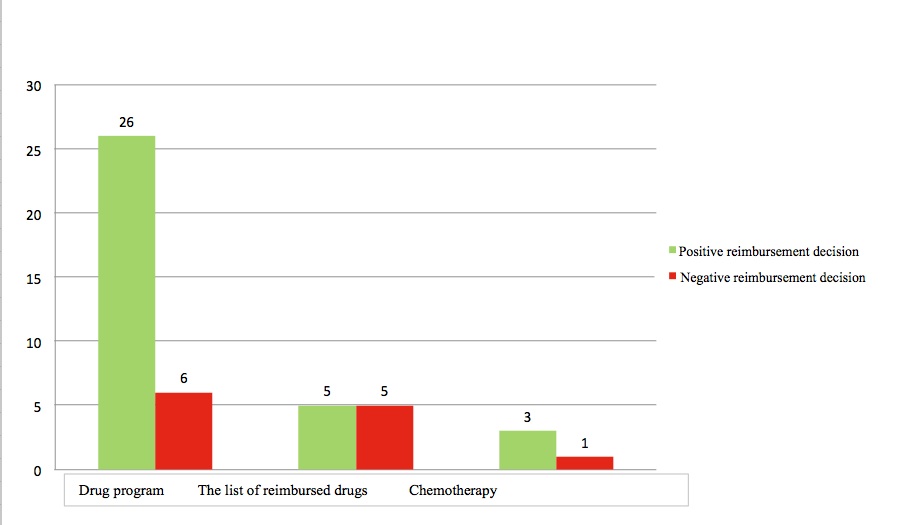
Most medicines that were positively recommended or recommended positively subject to some conditions are reimbursed from public funds, therefore available for the Polish patients. Only 12 products were denied reimbursement. In case of products applying to be reimbursed within drug program or chemotherapy catalog, the AHTAPol President’s positive recommendation translated into the positive reimbursement decision for most of them.
Figure 2. The number of AHTAPol positive recommendations
vs. the reimbursement decisions of the Minister of Health
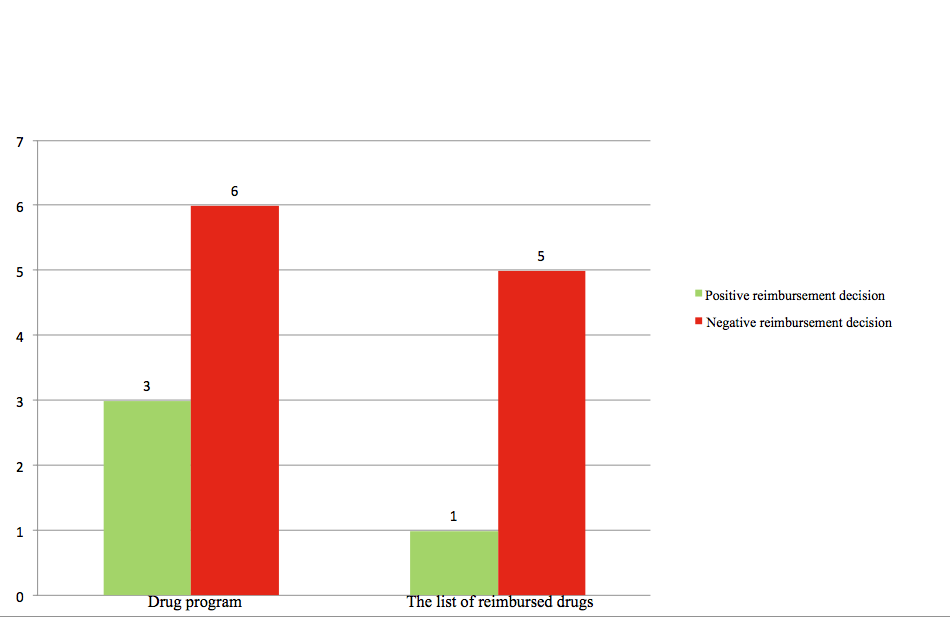
The analysis of negative recommendations showed that within the recommendations for drugs available through drug programs in 3 cases out of 6, whereas within the recommendations for drugs available through the register of reimbursed medicines, in 1 case out of 5, the Minister of Health decided to finance given products from public funds, despite negative recommendations (Figure 3).
Figure 3.The number of AHTAPol negative recommendations
vs. the reimbursement decisions of the Minister of Health
The following medicines were decided by the Minister of Health as eligible for reimbursement within drug programs: dasatinib, recommended for use in the chronic myelogenous leukemia treatment, palivizumab recommended for use in the RSV prophylaxis for children with bronchopulmonary dysplasia, and alglucosidase alfa recommended for use in the Pompe disease treatment [10,17,18].
In each recommendation for the above-mentioned products, there were objections to their economic aspects. Dasatinib and alglucosidase alpha turned out to be cost-ineffective, whereas palivizumab generated the rise in incremental expenditure for public payer. Furthermore, the AHTAPol President contested the effectiveness and safety of treatments using the above-mentioned medicines. It was observed that using dasatinib in the treatment category it was applying to, generates greater risk of side effects. The analyses for palivizumab did not demonstrate unequivocal clinical arguments to enlarge children’s group to have the medicine recommended, whereas in case of alglucosidase alpha there was provided insufficient scientific evidence that would confirm its impact on the patients’ life length and life quality. Despite these shortcomings, in case of alglucosidase alpha, the AHTAPol suggested that it is possible to reimburse this medicine under two conditions: that there are implemented the solutions proposed in the rationalization analysis, and that there is drawn an agreement for risk division. The fact that alglucosidase alpha was eventually decided eligible for reimbursement, indicates that the applicant agreed to these conditions in the course of their negotiations with the Economic Commission [17,18,19].
The Minister of Health also issued a positive reimbursement decision for tetrabenazine, used in the treatment of hyperkinetic motor disorders in Huntington’s disease. This medicine was negatively recommended by the AHTAPol President due to the two factors: its high treatment costs (the medicine was cost-ineffective for the price proposed by the applicant), which may be negotiated in the course of negotiations with the Economic Commission though, and the effectiveness and safety of treatment. It was highlighted in the recommendation that this medicine does not affect the natural course of the disease, and causes a number of side effects.
Based on the above-demonstrated line of argumentation, it appears that the AHTAPol President negative recommendation does not necessarily indicate a negative reimbursement decision, which further evidences that the Agency for Health Technology Assessment is a mere advisory body for the Minister of Health, and the final reimbursement decisions are taken by the Economic Commission and the Minister of Health themselves.
Discussion
Having analysed the material, one can conclude that the most positively recommended technologies received positive reimbursement decisions. It is worth to emphasize that receiving a negative recommendation does not imply the negative reimbursement decision, which confirms that recommendation of the AHTAPol President is only one of many aspects that are taken into account by the Minister of Health reimbursement decision making.
We can observe a growing role of risk-sharing in decision-making processes, almost all assessed application contain risk sharing scheme proposal. An option to use risk sharing schemes gives pharmaceutical companies a chance to propose better financial conditions to public payer keeping at the same time price confidential.
Among the limitations of the study we can list the fact that only 2012 year was analyzed with a small number of orders (132) assessed by AHTAPol in 2012. To compare, the number of Ministry of Health orders was 352 in 2013 and 292 in 2014. For this reason it would be reasonable to prepare analysis for subsequent years. Analysing the opinions, recommendations, and verifications, that were partly blinded due to data confidentiality can be regarded as another limitation of this study.
We have identified two studies on the similar subject. Both analyses were carried out for the period prior to the introduction of the Reimbursement Act. The first review covered 59 HTA recommendations from September 2007 until October 2008, and the second one referred to all recommendation (285 positions) issued before January 2011 [19,20]. In the first study 32 HTA evaluations received negative recommendation, 26 on the grounds of clinical evidence (insufficient clinical efficacy data and poor efficacy or safety was stressed) other 6 concerned non-clinical aspects such as unacceptable cost-effectiveness ratio (ICER), budget impact and risk of off-label use. 27 assessments received positive recommendations with different restrictions e.g. specific subpopulations, need for ICER improvement, lowering a price. ICER was above AHTAPoL threshold in 65% of positive recommendations and below the threshold in 44% of negative recommendations [19].
The aim of the second review was to answer whether ICER (cost per QALY) can be identified as a main criterion for AHTAPol decisions. Authors identified 177 positive and 108 negative recommendations. Clinical efficacy seemed to have the strongest impact on recommendations, but a positive influence on hard endpoints was also clearly reported in 15 negative recommendations and lack of such proven efficacy in 38 positive recommendations. Safety and cost-effectiveness aspects were more often recalled in negative than positive recommendations. According to the results of the study, for the analysed period, no threshold value of QALY as a clear indicator of a decision could be specified.
Based on these examples and our study we can conclude that decision making process regarding pricing and reimbursement is hardly predictable and is based on multi-dimensional process both before and after the Reimbursement Act was introduced.
Conclusions
The aim of this study was to analyze the innovative therapies availability for the Polish patients, after the Reimbursement Act being in force for over one year. Based on the analyzed material, i.e. recommendations and reimbursement decisions, it appears that the Polish patients do not have their access to new technologies limited. Most of the positively recommended technologies received positive reimbursement decisions. It is worth to emphasize that receiving a negative recommendation does not imply the negative reimbursement decision. On the one hand, this can be perceived as an example of insufficient transparency of the reimbursement process. On the other hand however, since the negative recommendation does not end the reimbursement process, this can be perceived as an opportunity for the Polish patients to have a wider access to innovative therapies.
- Ustawa z dnia 12 maja 2011 r. o refundacji leków, środków spożywczych specjalnego przeznaczenia żywieniowego oraz wyrobów medycznych (Dz.U. 2011 nr 122 poz. 696)
- Rekomendacja nr 9/2013 z dnia 28 stycznia2013 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Ruconestkonestat alfa, w ramach programu lekowego: leczenie ostrego dziedzicznego obrzęku naczynioruchowego (ICD-10 D84.1) konestatem alfa (Ruconest); Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/114/REK/RP_9_2013_Ruconest.pdf
- Stanowisko Rady Przejrzystości nr 19/2013z dnia 28stycznia 2013 r.w sprawie zasadności finansowania leku Ruconest (konestat alfa)w ramach programu lekowego„Leczenie ostrego dziedzicznego obrzęku naczynioruchowego (ICD-10 D.84.1) konestatem alfa (Ruconest) ”; Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/114/SRP/U_3_38_130128_stanowisko_19_Ruconest%28konestatalfa%29_obrzek.pdf
- Rekomendacja nr 104/2012 z dnia 12 listopada 2012 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacja produktu leczniczego Procoralan we wskazaniu: przewlekła niewydolność serca II do IV stopnia wg klasyfikacji NYHA, z zaburzeniami czynności skurczowej, u pacjentów z rytmem zatokowym, u których częstość akcji serca wynosi >= 75 uderzeń na minutę, w skojarzeniu z leczeniem standardowym, w tym z beta-adrenolitykami lub gdy leczenie beta-adrolitykiem jest przeciwwskazane albo nie jest tolerowane. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/086/REK/RP_104_2012_iwabradyna_5_mg.pdf
- Rekomendacja nr 21/2012 Prezesa Agencji Oceny Technologii Medycznych z dnia 11 czerwca 2011 r. w sprawie objęcia refundacją środka spożywczego specjalnego przeznaczenia żywieniowego Nutramigen 1 LGG, preparat złożony, 400g w puszce, we wskazaniach: alergia na białko mleka krowiego, inne alergie pokarmowe, objawy związane z alergia pokarmową, nietolerancja laktozy, wtórna nietolerancja sacharozy, dodatni wywiad rodzinny w kierunku chorób alergicznych. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/028/REK/RP_21_2012_Nutramigen_1_LGG.pdf
- Rekomendacja nr 38/2012 Prezesa Agencji Oceny Technologii Medycznych Z dnia13sierpnia2012r.w sprawie objęcia refundacją produktu leczniczego Etruzil 2,5 mg x30 tabl.,EAN 5909990710201 we wskazaniu: wczesny rak piersi w I rzucie hormonoterapii. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/036/REK/RP_38_2012_Etruzil.pdf
- Rekomendacja nr 106/2012z dnia 12 listopada 2012 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Thalidomide Celgene (talidomid) kapsułki twarde, 50 mg, 28 szt. (2 blistry po 14 kapsułek), EAN 5909990652976 we wskazaniu: Thalidomide Celgene w połączeniu z melfalanem i prednizonem w leczeni U pierwszego rzutu nieleczonego szpiczaka mnogiego u pacjentów w wieku ≥ 65 lat lub u pacjentów niekwalifikujących się do chemioterapii wysokodawkowej. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/082/REK/RP_106_2012_Talidomid.pdf
- Rekomendacja nr 127 /2012 z dnia 18 grudnia 2012 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Evoltra (klofarabina), koncentrat do sporządzania roztworu do infuzji 1 mg/ml (20 mg/20 ml), EAN 5909990710997 we wskazaniu: leczenie ostrej białaczki limfoblastycznej (ALL) u dzieci i młodzieży z nawrotem lub oporną na leczenie chorobą po zastosowaniu przynajmniej dwóch wcześniejszych standardowych cykli i w przypadku, gdy brak innych opcji pozwalających na przewidywanie długotrwałej odpowiedzi, u chorych kwalifikujących się do przeszczepu macierzystych komórek krwiotwórczych. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/035/REK/RP_127_2012_Evoltra.pdf
- Rekomendacja nr 74/2012 z dnia 1 października 2012 r.Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Revolade (Eltrombopag), tabletki powlekane, 25 mg, 28 tabl., kod EAN: 5909990748204; we wskazaniu : leczenie dorosłych chorych na pierwotną małopłytkowość immunologiczną. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/074/REK/RP_74_2012_25_mg_Revolade.pdf
- Rekomendacja nr 8/2013 z dnia 28 stycznia 2013r.Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Myozyme (alglukozydaza alfa) w ramach programu lekowego: leczenie choroby Pompego ICD-10 E74.0. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/107/REK/RP_8_2013_Myozyme.pdf
- Rekomendacja 51/2012 Prezesa Agencji Oceny Technologii Medycznych z dnia 3 września 2012 r. w sprawie objęcia refundacją produktu leczniczego XGEVA (denosumab) 120 mg, 1,7 ml roztworu do wstrzykiwań we wskazaniu:" zapobieganie powikłaniom kostnym (SRE) u pacjentów z rakiem gruczołu krokowego z przerzutami do kości”. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/029/REK/RP_51_2012_Xgeva.pdf
- Rekomendacja nr 72/2012 Prezesa Agencji Oceny Technologii Medycznych z dnia 24września 2012r.w sprawie objęcia refundacją produktu leczniczego Tetmodis, 25mgx112tabletek,112tabl.,kodEAN5909990805594wewskazaniuhiperkinetyczne zaburzenia motoryczne w chorobie Huntingtona. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/041/REK/RP_72_2012_Tetmodis.pdf Available from:
- Rekomendacja nr 81/2012 Prezesa Agencji Oceny Technologii Medycznych z dnia 15 października 2012 r. w sprawie objęcia refundacją produktu leczniczego Primacor, chlorowodorek lerkanidypiny, tabletki powlekane 10 mg, 60 szt., kod EAN 5909990801886; we wskazaniu: leczenie łagodnego lub umiarkowanego samoistnego nadciśnienia tętniczego. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/031/REK/RP_81_2012_Primacor.pdf
- Rekomendacja nr 42/2012 z dnia 22 sierpnia 2012 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Neupro, rotygotyna, plastry, 4 mg/24 h, 7 plastrów, EAN 5909990587636 we wskazaniu wynikającym z wniosku refundacyjnego: leczenie pacjentów z zaawansowaną chorobą Parkinsona, u których występują powikłania motoryczne i/lub dyskinezy związane ze stosowaniem lewodopy. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/047/REK/RP_42_2012_Neupro.pdf
- Rekomendacja nr 108/2012 z dnia 12 listopada 2012 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Levetiracetam Teva (levetiracetamum); tabl. powl.: 250 mg; 100 szt.; kod EAN 5909990879106 we wskazaniu: monoterapia w leczeniu napadów częściowych lub częściowych wtórnie uogólnionych u pacjentów w wieku od 16 lat z nowo rozpoznaną padaczką. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/085/REK/RP_108_2012_Levetiracetam.pdf
- Rekomendacja nr 7/2013 z dnia 21 stycznia 2013 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Levetiracetam GSK (levetiracetam) we wskazaniu: leczenie padaczki w I rzucie. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/097/REK/RP_7_2013_Lewetiracetam.pdf
- Rekomendacja nr 70/2012 Prezesa Agencji Oceny Technologii Medycznych z dnia 17 września 2012 r. w sprawie objęcia refundacją produktu leczniczego Synagis (paliwizumab) proszek i rozpuszczalnik do sporządzania roztworu do wstrzykiwań, 50 mg, 1 fiol. 50 mg proszku + 1 amp. 0,6 ml rozp. (100 mg/ml), EAN 5909990815616 w ramach programu lekowego: “Profilaktyka zakażeń wirusem RS u dzieci z przewlekłą chorobą płuc (dysplazją oskrzelowo-płucną)". Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/066/REK/RP_63_2012_Sprycel.pdf
- Rekomendacja nr 8/2013 z dnia 28 stycznia 2013 r. Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Myozyme (alglukozydaza alfa) w ramach programu lekowego: leczenie choroby Pompego ICD‐10 E74.0. Available from: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2012/068/REK/RP_70_2012_Synagis.pdf
- Kolasa K. Review of HTA recommendations for drug therapies in Poland issued from September 6, 2007 until October 28, 2008 by the Consultative Council (appraisal committee) of AHTAPol in Poland. Value in Health, May 2009 Volume 12, Issue 3: A94
- Niewada M., Polkowska MA., Jakubczyk M., Golicki D., What determines the recommendations issued by Polish Health Technology Agency (AHTAPol)?, Value in Health 14 (2011): A241