Hepatitis C – the implications and the need for change in the health care system in Poland
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Hepatitis C is a disease spreading across the world. It is estimated that more than 185 million people are potentially infected with HCV. Each year approximately 350 thousand people die of diseases resulting from HCV. In Poland about 231 thousand people are believed to be infected, with up to 85% of them unaware of their progressive illness. Hepatitis C leads to serious complications in the liver and, consequently, can lead to death. The disease generates appreciable costs associated with the treatment of the complications of HCV in the advanced stages of the disease as well as lost productivity. As there is no vaccine against HCV, hepatitis C treatment is the only way to eradicate the virus. This is now possible with new therapies. This article presents the burden of hepatitis C and its changing dynamics in the context of hepatitis B and HIV in Poland. The natural history, the costs generated by the disease, available current therapies and suggested ways forward are also discussed in the article.
Introduction
Hepatitis C is an infectious disease caused by the Hepatitis C Virus (HCV), which mainly affects the liver. In the first stage, there is progression of acute hepatitis with necrotic and inflammatory changes rapidly developing in the liver. Acute hepatitis C is asymptomatic in approximately 70-80% cases. Spontaneous elimination of HCV occurs in 14-46 % of patients, mainly in symptomatic patients [1]. If the virus is present in the body for longer than six months, the disease progresses into chronic hepatitis. Chronic hepatitis is characterized by multiple necrotic and inflammatory lesions, which are typically asymptomatic. This is the cause of serious complications in the liver and may lead to hepatocellular carcinoma and eventually to the death of the infected person if left untreated. It is estimated that approximately 85% of patients with HCV are unaware of their disease [2,3]. In these patients the destructive processes take place covertly resulting in patients being vulnerable to the serious complications of the disease and at the same time being a reservoir for the virus, allowing further spread of the virus [4]. These features make HCV a serious public health problem if left untreated; alternatively there are a few preventative measures in place, which will be discussed in this article.
The problem of HCV infection and hepatitis C is recognised by international organizations, including the World Health Organization (WH) and the European Union Parliament [5,6]. WHO estimates that approximately 2.8% of the global population has anti-HCV antibodies, which translates into more than 185 million people potentially infected. In 10-15% of patients with chronic hepatitis C, cirrhosis of the liver occurs in approximately 20 years' time after the initial infection if left untreated. This can lead to further complications including liver failure and hepatocellular carcinoma, which can result in premature death. Each year approximately 350 thousand people globally die of diseases resulting from HCV infection. [5,7] In developed countries, including Poland, the number of deaths caused by hepatitis C now exceed the number of deaths resulting from infection with HBV (Hepatitis B Virus, which causes hepatitis B) or HIV (Human Immunodeficiency Virus) [8,9].
The majority of HCV cases currently observed in Western Europe have been detected among drug injection users [10]. In Poland, however, the dominating route of transmission is iatrogenic. Minor medical procedures are responsible for up to 80% of all the infections [11,12]. In comparison to other European countries, Poland is characterized by a relatively low detection rate for hepatitis C [2]. The detection of HCV infection usually occurs by chance, e.g. during the pre-donation screening, or when the patient reports to the physician with symptoms of liver disease at an advanced stage. A low level of knowledge about HCV and hepatitis C, as well as the routes of virus transmission, are factors resulting in new infections [13]. This low level of knowledge currently translates into a small number of diagnostic measures being undertaken and the lack of interest of interest among national and regional authorities to implement preventive actions. No less important are the economic consequences of HCV infection, involving the direct costs of treating the disease including its complications as well as indirect costs resulting from reduced productivity and premature withdrawal of patients from the labour market. For these reasons it is increasingly critical for the various authorities in Poland to make changes to the current health policies to reverse this growing trend of new cases of HCV infection and deaths caused by hepatitis C.
Epidemiology
In patients in whom the disease process is active or has ended, anti-HCV antibodies are detectable. This is used for the initial diagnosis of hepatitis C. To confirm the active viremia it is necessary to determine the genetic material of the virus (HCV RNA) in the blood serum of the patient [11].
As previously mentioned, the WHO estimates that approximately 2.8% of the global population has anti-HCV antibodies, which translates into more than 185 million potentially infected globally. As mentioned, the number of people dying annually of diseases resulting from HCV infection is approximately 350 thousand people; however, this figure will grow in the coming years if patients are untreated and there is a lack of preventative and other measures in place among countries [7,14].
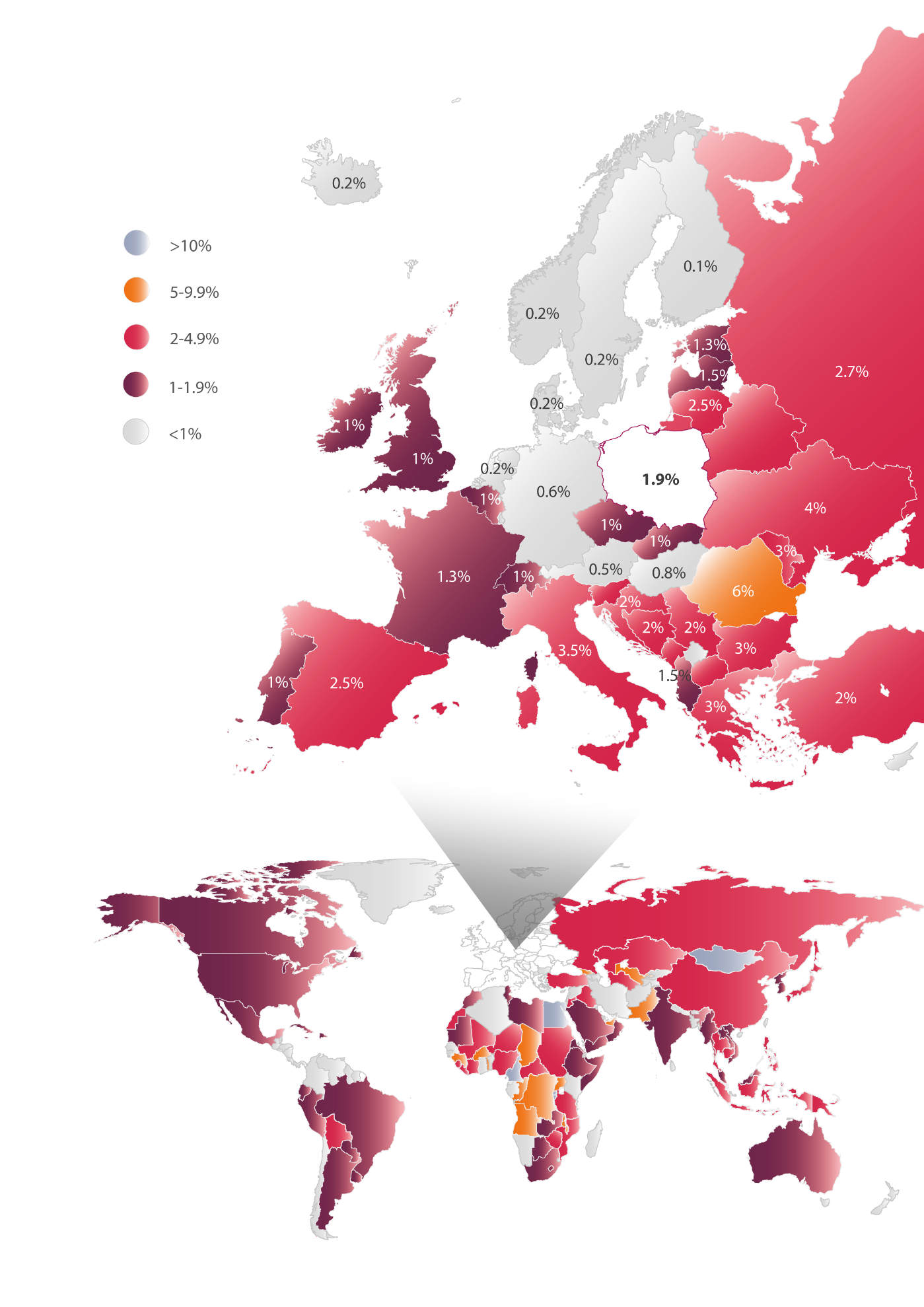
In Europe, the prevalence of HCV ranges from 0.1% to 6% depending on the country. Comparison of the epidemiological status in different countries poses some difficulties due to variations in diagnostic procedures, various definitions of infection, different methodologies used in the various studies, as well as the time when the epidemiological research took place. According to current estimates, the lowest prevalence of the virus (0.1-1%) is in northern Europe. These infections are mainly present among people aged 30-50 years, most frequently associated with needle sharing by intravenous drug users. Higher prevalence rates of HCV, e.g. 2.5-3.5%, are seen in southern Europe, e.g. Spain, Italy, Greece, where in addition to infections transmitted by the intravenous route, a substantial number of infections among people aged over 50 years of age as due to the iatrogenic route of transmission, i.e. they became infected in hospitals. The highest prevalence of the virus ranging from 1.3% to 6% is observed in Eastern Europe, where the infections take place most frequently via the hospital route, i.e. among recipients of blood and organ donation as well as other causes (Figure 1) [15].
Poland appears to have an average ratio of HCV prevalence based on official data with all limitations. Epidemiological studies [16,17], carried out between 2009-2011 and covering 30 thousand patients indicate that the presence of anti-HCV antibodies is approximately 1.9% of the population, which corresponds to approximately 732 thousand people in Poland. However, the complete testing procedure requires a double repetition of the diagnostic approach. Epidemiological studies that use this method indicate that the prevalence of anti-HCV in Poland at a level of 0.95%, translating into approximately 366 thousand people in Poland having contact with the virus in their lifetime. Moreover, to confirm active infection, it is necessary to demonstrate the presence of viral genetic material. The percentage of the Polish population who present with both antibodies and genetic material of the virus is at a level of 0.6%, translating into 231 thousand people with active infection [16,17]. Meanwhile, the population of hepatitis B in Poland is estimated at 1-1.5% (400 to 600 thousand people) [18,19], while those with HIV infection is estimated at 0.07-0.12%, i.e. 27 to 46 thousand people) [29].
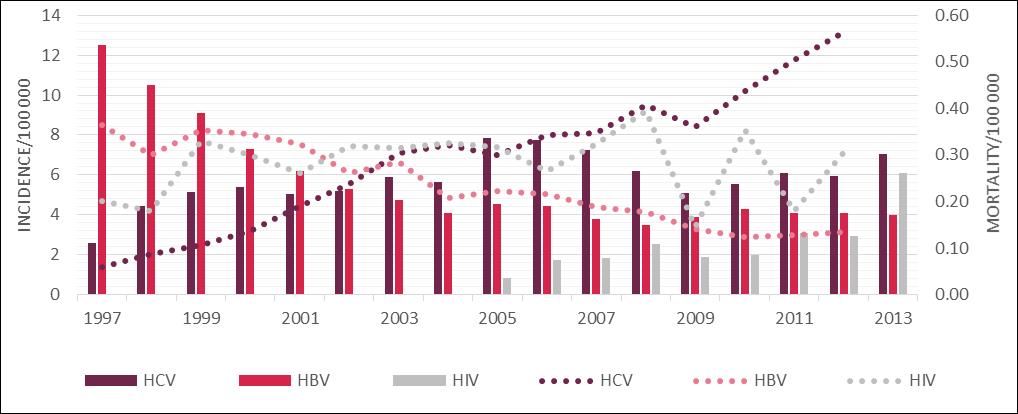
The epidemiology of hepatitis C in Poland has been monitored by the National Institute of Public Health - National Institute of Hygiene (NIPH - NIH) since 1997. However, the number of registered cases is likely to be lower than the actual number of infections due to the current low level of reportability. [20] Consequently, this data fails to show the accurate epidemiological situation of hepatitis C in Poland. Bearing this in mind, between 1997 and 2013 NIPH - NIH registered approximately 38 thousand people with HCV infection in Poland [21]. However as stated, approximately 85% of people with hepatitis C remain unaware of their infection explaining the current low rates reported by NIPH-NPH. In the first year of the monitoring, the number of registered cases stood at approximately 1 thousand. In the following year this number was almost doubled, and in 1998-2004 remained at 2 to 2.2 thousand patients. In 2005 there was a sudden increase to approximately 3 thousand recorded cases. The explanation of this phenomenon may be an increase of the public awareness of HCV and an increase in the number of performed diagnostic tests. [11] After a period of growth, the number of registered cases declined in 2009 to the level seen between 1998 to 2004. However, currently there is an upward trend with a total of 2705 new HCV infections reported in 2013. It means that there were 7.03 HCV infections per 100 thousand Poles [21].
Meanwhile from the 1980s, thanks to the introduction of a universal system of vaccination for newborns and adolescents, the number of people infected with HBV has been systematically decreasing. In 2013 the number of newly registered cases of hepatitis B in Poland was 1 540 (4.00 per 100 thousand people). Newly registered HIV cases were, in turn, 1163 (3.02 per 100 thousand people) [21].
According to the Central Statistical Office of Poland, the number of deaths due to HCV is systematically increasing. In 2012, 217 people died of hepatitis C in Poland (0.13 per 100 thousand people). That is a 5-fold increase compared to the end of the 1990s. It was also more than the number of deaths caused by infections with HBV or HIV (Figure 2). The number of deaths in 2012 caused by hepatitis B in Poland was 52 (0.13 per 100 thousand people) and AIDS, which is a consequence of HIV infection, 118 (0.31 per 100 thousand people).
The dynamics of the HCV, HBV and HIV epidemiological situation in Poland in relation to hepatitis C virus in the context of hepatitis B and HIV [21,63]
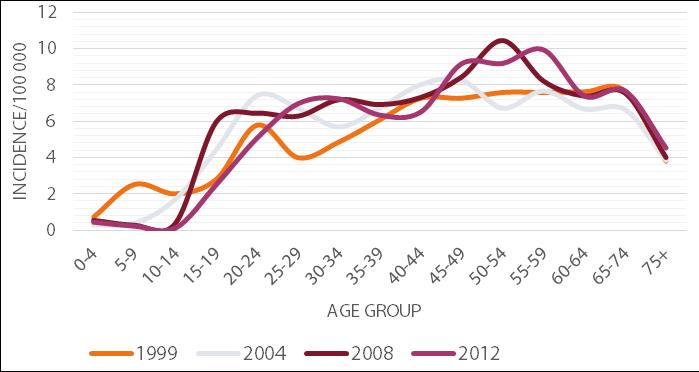
According to the latest NIPH - NIH data, the majority of the new cases of hepatitis C is observed in the age group 45-59. However, an appreciable number of infections is also recorded in the age groups 25-44 and 60-74 (Figure 3).
This relationship has been present in Poland consistently since the 1990s. In the case of younger patients, infection is usually detected accidentally, e.g. during pre-donation screening. HCV infection can result from an injectable drug episode or from minor procedures breaking the continuity of the tissue, e.g. medical procedures, piercing, tattooing or scarification. In contrast, infections in the elderly are mostly detected during a visit to the physician, caused by the symptoms of developed hepatitis. The infection is most often the result of hospitalizations received before 1990, when the rules for the prevention of transmission of infection were not as restrictive as currently exist, or as a result of a blood transfusion before 1993, when the blood was not routinely tested for the presence of HCV [22]. In addition, men are more often affected by hepatitis C than women. In 2012, the incidence rate in Poland for males was 6.60 per 100 thousand and for women 5.34 per 100 thousand. Moreover, the infection is detected nearly twice as often among urban residents compared with rural ones. In 2012 in Poland, the incidence rates for urban and rural areas respectively were 7.25 and 3.95 per 100 thousand population [21].
HCV exhibits considerable genetic variability. There are six major genotypes of HCV, among which several subtypes can be distinguished. The genotype 7 was also detected, but to date it has been poorly investigated [23]. The prevalence of HCV genotypes varies depending on the geographical area. Genotypes 1-3 occur worldwide, genotype 4 dominates in the Middle East, Central Africa and Egypt, genotype 5 occurs primarily in South Africa, and genotype 6 in Asia. [24] In the extensive epidemiological study conducted by Panasiuk et al. in 2013 [25], it was indicated that 86% of HCV infections in Poland in 2011-2012 were genotype 1.
Course and consequences of the disease
Because HCV is a blood-borne virus, the infection can only occur through exposure to infected blood. Most often the infection happens during medical procedures and among intravenous drugs users, and are more probable than vertical infections [11,22].
In the second half of the twentieth century, infections in Europe occurred mainly during medical procedures breaking the continuity of the tissues, such as surgical and dental injections and other invasive procedures, as well as through blood transfusions and organ transplants [22]. In the early 1990s after the discovery of HCV in 1989, blood and organ donors began to be examined for the presence of HCV antibodies and viral genetic material. The introduction of new procedures increased the safety of the patients, e.g. appropriate methods for HCV sterilization. This caused a gradual decline in the number of new iatrogenic infections [22,26]. At the end of the twentieth century, infections among intravenous drug users became more common as a result of the sharing of contaminated injecting equipment. This route is the cause of 80% of the registered cases of HCV infection in Europe in 2011 [10]. Vertical infections (from mother to child) are also present, most likely perinatal. It is estimated that vertical infections represent 3-10% of all infections in the countries of the European Economic Area [22]. Other possible, but less likely routes of transmission of HCV, may result from such practices as tattooing, piercing, scarification, acupuncture or cosmetic procedures. Infections which result from a day to day contact with an infected person are scarce. In such cases, the transmission of the virus can result from the shared use of personal items such as razors, toothbrushes or nail care accessories contaminated with the HCV infected blood [27]. Infections through sexual intercourse are possible, but very rare. In this case the infection occurs when the mucous membranes continuity is broken [22,28].
In developed countries in Europe 30-60% (depending on the country) of people living with HCV have been infected as a result of intravenous drug use [39]. In Poland, the majority of infections occur during small invasive medical procedures during where safety procedures have been neglected. According to specialists' opinion, iatrogenic events may currently be the cause of 80% of the infections in Poland [11,12].
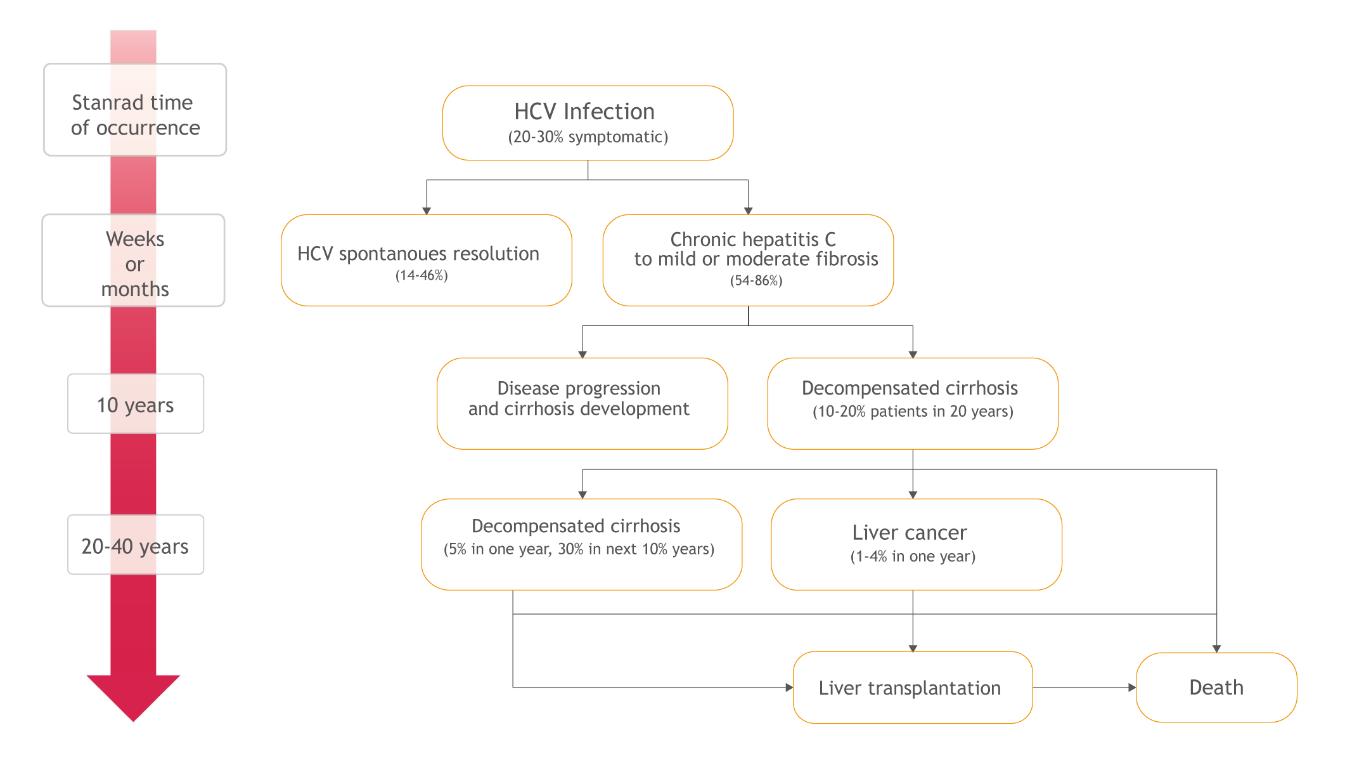
Initially, hepatitis C usually develops asymptomatically and the ill person is unaware of when and how the infection happened. Therefore, there are inconsistencies related with the description of the natural history of the disease and the average life of the patient is difficult to estimate. However, it is known that the first stage of the illness is acute hepatitis, which is typically asymptomatic, which progresses into the chronic form in between 54 to 86% of cases. Chronic hepatitis, in turn, can run for years without noticeable symptoms, but with accompanied progressive fibrosis of the liver, which may lead to serious complications and potentially to premature death (Figure 4) [1,4,29].
Chronic hepatitis C is accompanied by the presence of genetic material of the virus in the blood for more than 6 months and, as mentioned, develops in between 54 to 86% of patients with acute infections [1]. The probability of the transition to the chronic condition depends on many factors including age, sex, ethnicity, and the course of the preceding acute phase. Spontaneous elimination of HCV at this stage of the disease is sporadic - 0.02% per year. Chronic hepatitis C may be asymptomatic for a long time. However, as a result of the inflammation in this phase, progressive liver damage and impairment of its function occur [29].
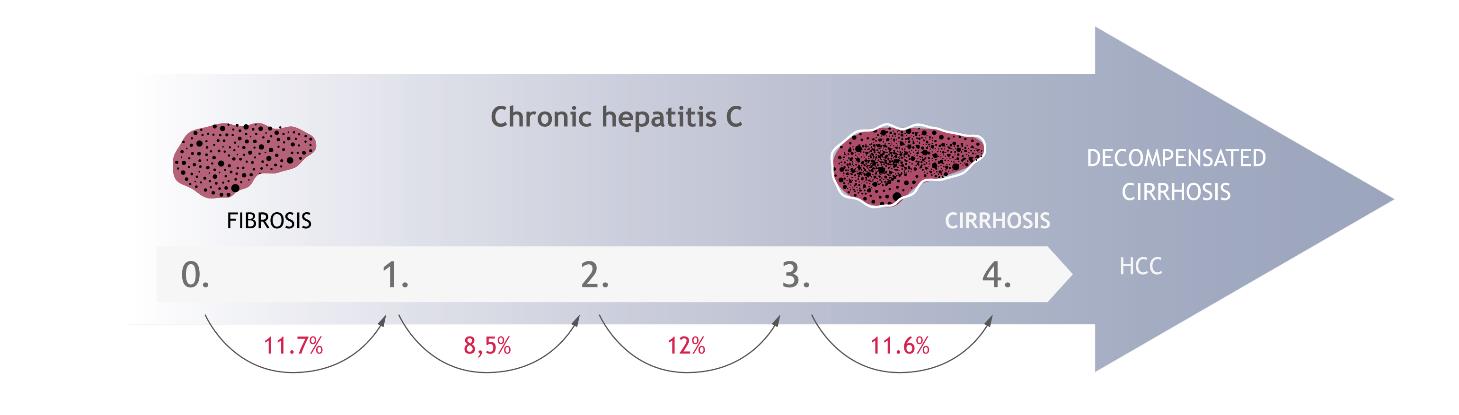
The development of disease is associated with progression of liver fibrosis. This process involves replacing the damaged liver tissue elements by connective tissue as a result of ongoing inflammation. Fibrosis leads to the remodelling of the organ and to a significant impairment of its functions. In the early stages, fibrosis develops in the portal and periportal areas. After this, it extends from one area to another one, forming the so-called fibrosis bridges. In the final stages, cirrhosis of the organ occurs. The severity of the liver damage is assessed by performing a biopsy or other equivalent non-invasive methods e.g. Fibrotest or FibroScan, and is determined by using a 5-point scale, where 0 means no change, and 4 - the most severe changes, i.e. cirrhosis. The rate of changes depends on many factors including the age and sex of the infected person, alcohol consumption and the presence of comorbid conditions [29]. However, it is estimated that the development of fibrosis from the stage 0 to 1 occurs in 11.7% of patients within a year of infection. The annual probability of progression to the next stages is 8.5%, 12.0% and 11.6%, respectively [30] (Figure 5). In Poland, patients are mostly diagnosed in the first or the second stage of liver fibrosis (approximately 70%) [31–33].
The annual probability of transition to the subsequent stages of liver fibrosis in patients with hepatitis C [30]
Cirrhosis is the final stage of liver fibrosis, which occurs in 10-15% of patients with chronic hepatitis C within 20 years from the initial infection. Firstly, HCV may give no symptoms and the liver can function properly. This condition is referred to as a compensated liver cirrhosis. The survival rates for patients with compensated cirrhosis at 3, 5 and 10 years post infection are 96%, 91% and 79% respectively [29]. However, 5% of patients per year and 30% of the patients per 10 years experience decompensation of cirrhosis, which leads to severe complications, such as ascites, bleeding in the upper gastrointestinal tract (as a result of esophageal varices or portal gastropathy) or hepatic encephalopathy [29]. The probability of 5-year survival with decompensated cirrhosis of the liver is much lower, equalling 50% of the cases. It is believed that decompensated cirrhosis is the most common cause of death as a direct consequences of hepatitis C [29,34]. Another consequence of chronic hepatitis C is hepatocellular carcinoma (HCC), which affects 1-4% patients with HCV per year. It is estimated that the risk of this cancer is 17 times higher in the case of HCV infection than in cases of uninfected patients. Apart from the consumption of alcohol (20% of HCC) and HBV infection (10-15%), HCV is the main factor favouring the development of this cancer in Europe (60-70%) [35]. The average time that elapses from initial infection to the development of HCC is almost 30 years [29]. This cancer is poorly predictive and in most cases leads to death within a few years from diagnosis. It is estimated that the probability of death for people diagnosed with hepatocellular carcinoma and HCV infection is 41-46% per year [36–38].
Advanced cirrhosis and liver failure are the reasons for liver transplantation in many patients. Hepatitis C is the leading cause of the liver transplantation in Poland [39,40] and in other countries, for example, in the USA [41]. According to the Polish data, HCV infection or coinfection is responsible for 23-28% of all liver transplantations [42]. Despite the growing number of transplantations, there is still unmet demand at approximately double the number of transplantations undertaken. While the number of grafts resulting from post-inflammatory HCV cirrhosis decreases, the number of transplantations in patients with hepatocellular carcinoma will increase [43]. Unfortunately, the relapse of hepatitis C in patients after the liver transplantation is inevitable, resulting in recirrhosis in 25% of cases within 5 years, which may be the reason for retransplantation [43,44].
Besides the serious complications of liver, hepatitis C may result in extrahepatic disorders, occurring in 1-2% of the infected with HCV. The most common extrahepatic complication is cryoglobulinemia, resulting in symptoms such as fatigue, skin rash, bleeding disorder, arthralgia, Raynaud's symptoms, inflammation of the blood vessels, kidney disorders or peripheral neuropathy. Other observed disorders are membrane-proliferative glomerulonephritis, porphyria cutaneous tarda, lichen planus and vitiligo. In the course of hepatitis C lymphoma and Hodgkin's lymphoma, autoimmune thyroiditis, Sjogren's syndrome and seronegative arthritis are also observed. It is unclear whether these disorders are directly caused by HCV infection or by a chronically stimulated immune response triggered by the inflammation [29].
Treatment
Hepatitis C is a disease that can be treated effectively. The main goal of the therapy is to achieve a sustained virological response (SVR) to prevent hepatic complications and reduce the mortality by eliminating HCV from the body of the patient [45]. Due to the lack of vaccines against HCV, treating hepatitis C is, in fact, the only way in which the reservoir of potential infections can be reduced [11].
So far, the standard treatment for all patients with chronic hepatitis C has been a non-specific therapy, which consists of stimulating the immune system to the antiviral response for the HCV present in the organism. A combination of pegylated interferon alpha with ribavirin (PegINFα + RBV) has been used as a nonspecific dual therapy. [46] Interferon is responsible for stimulation of the antiviral response while in vitro research has confirmed the activity of ribavirin against some RNA and DNA viruses. Pegylated interferon alpha is administered as a weekly injections and ribavirin is taken orally twice a day. The total duration of therapy can last up to 72 weeks [47–49]. The efficacy of dual therapy PegINFα + RBV strongly depends on the genotype of the virus. For genotypes 2 and 3, an effective cure is achieved in 87% and 77% of patients, respectively [8]. The percentage drops to approximately 56% in the case of genotype 4 [50–52]. In the case of genotype 1, identified in nearly 90% of patients in Poland [25], the effective treatment is achieved in only 45% of cases [8]. The use of interferon and ribavirin is associated with serious side effects [53]. The most important side-effects include depression, suicide attempts, anorexia, nausea, anaemia and flulike symptoms [14,48,49]. These side-effects can result in discontinuation of treatment, with estimates that less than 50% of patients complete a course of treatment [46]. This is important as effective therapy requires patients taking at least 80% of the indicated doses for at least 80% of therapy duration [47]. There are also contraindications for combination therapy PegINFα + RBV limiting the population patient population for treatment [48,49].
The second group of drugs used in the treatment of chronic hepatitis C are specific drugs pointed directly against the virus. They are inhibitors of enzymes involved in virus replication. Currently their function is the direct intervention in the process of HCV multiplication. The following drugs are currently registered in Poland: first-generation NS3/4A protease inhibitors boceprevir, telaprevir and second-generation direct-acting antiviral agents sofosbuvir, simeprevir, daclatasvir, ledipasvir tablets [54–59] and recently ombitasvir, paritaprevir, ritonavir and dasabuvir. [60] Many other new substances are in the process of drug registration or at advanced levels of clinical trials.
Boceprevir and telaprevir are aimed at patients infected with the genotype 1. Simeprevir has been tested in patients infected with genotypes 1 and 4 while sofosbuvir can be used for infection of genotypes 1-6. In a population with genotype 1, all drugs are routinely used together with pegylated interferon alpha and ribavirin, in so-called triple therapy. Boceprevir is administered orally, three times a day, telaprevir twice to three times a day, while simeprevir and sofosbuvir are administered once a day. The total duration of the therapy in the case of boceprevir and telaprevir is 48 weeks. Treatment with simeprevir takes less time - 24 weeks, and with sofosbuvir only 12 weeks [54–57]. The use of boceprevir or telaprevir with interferon and ribavirin in naive patients allows to obtain a sustained response at the level of 54-75%, and in patients undergoing treatment again, because of the failure of previous PegINFα + RBV treatment, effectiveness of this therapy is up to 51-67% [61]. Simeprevirand sofosbuvir used in combination with interferon and ribavirin exhibit higher efficiency with cure rates up to 80% and 95% in naive patients [14,46]. It needs to be stressed that sofosbuvir may also be given to patients not eligible for treatment with the use of interferon. In such case it is only used in combination with ribavirin, but then the efficiency is at a level of 50-84%, and the treatment must be extended to 24 weeks [62]. In this group of patients it is also possible to use combination therapy consisting of two specific drugs - sofosbuvir and simeprevir, and optionally ribavirin. Preliminary data indicate that the response to that treatment can be over 90% [63–65]. However, triple therapy, besides the higher efficiency, has the same limitations associated with the comfort and safety as a dual therapy. Patients are still exposed to the adverse events resulting from the use of interferon and ribavirin. Furthermore, especially in the case of boceprevir and telaprevir, there is an additional risk of side effects [14,54,55].
The therapy with combination of ledispavir and sofosbuvir administered for 8 or 12 weeks with or without ribavirin is associated with even higher rate of sustained virological response at a level of 93-96% among previously untreated patients with HCV genotype 1 infection without cirrhosis [66]. The most advanced available therapy with ombitasvir, paritaprevir and ritonavir in combination with dasabuvir is approved for the treatment of genotype 1 chronic hepatitis C virus infection, including patients with compensated cirrhosis. This therapy is dosed orally twice daily for 12 weeks taken with or without ribavirin, except patients with cirrhosis, who should take it for 24 weeks and is effective at a level of 95-100% [60]. For the treatment of genotype 4 chronic hepatitis C patients, this treatment consists of ombitasvir, paritaprevir and ritonavir taken with ribavirin and effectiveness of this therapy is up to 100% [60].
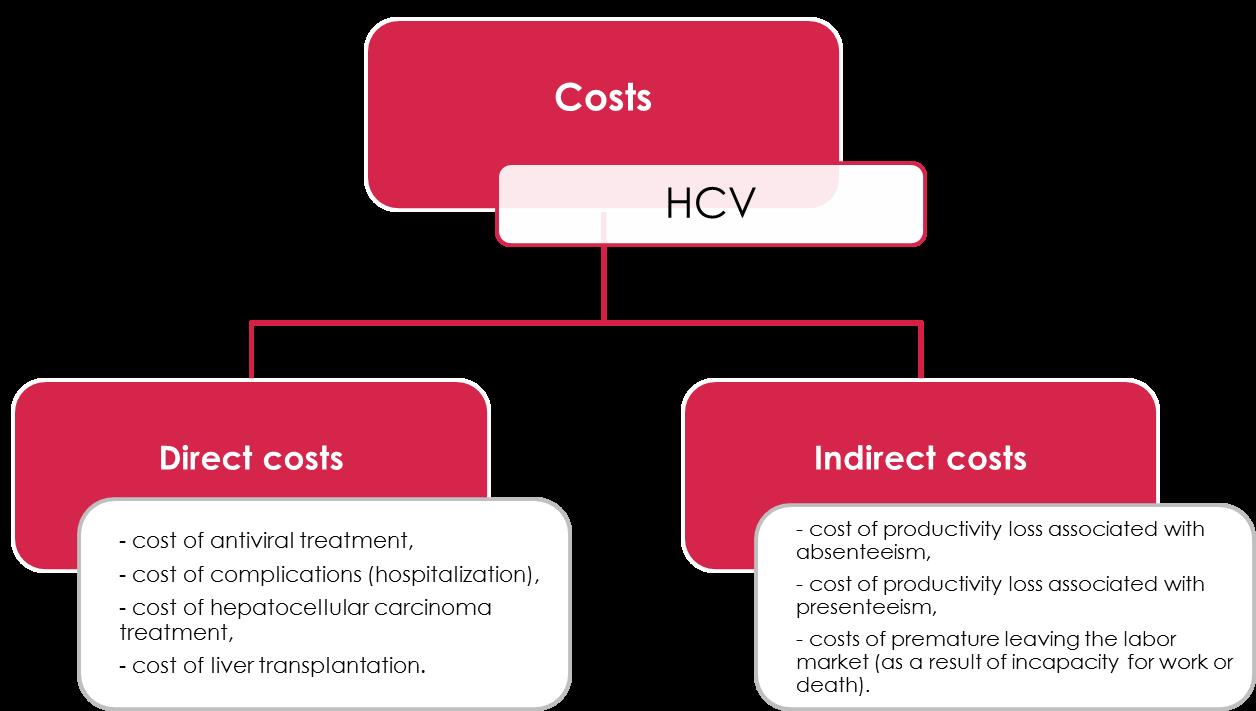
Costs – direct and indirect
There are two types of costs: direct and indirect, (Figure 6) and vary depending on HCV stage (Figure 7).
Estimated direct costs associated with hepatitis C treatment in 2013 was 234 million PLN, while annual costs associated with productivity loss due to deaths, permanent incapacity for work, absenteeism and presenteeism computed with the conservative approached 585 million PLN taking into account only currently treated patients and maximum estimation of presenteeism.
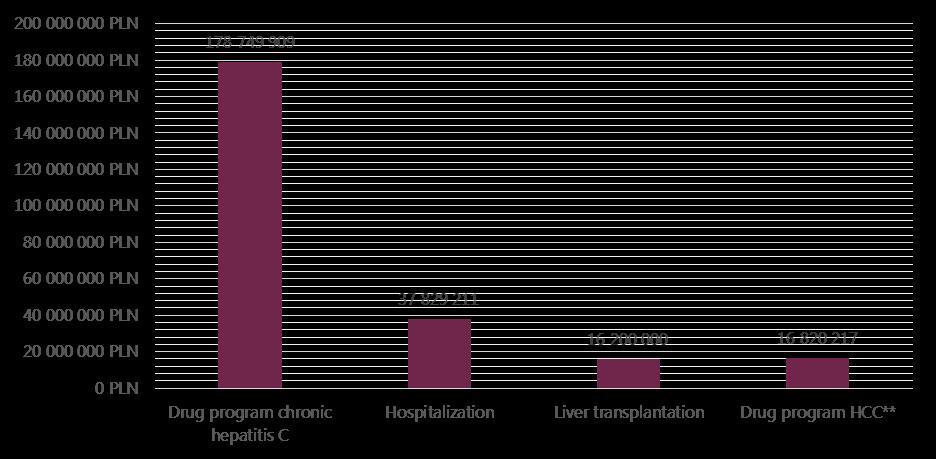
Direct costs are calculated as the sum of all health services during one year based on individual data. The largest component of costs per person associated with chronic hepatitis C are for the treatment of severe complications. The estimated cost of one liver transplantation is 200 thousand PLN and annual post-liver transplantation costs are 20 thousand PLN. Costs of drugs during the six months of hepatocellular carcinoma drug program are estimated at 99 thousand PLN per patient (Figure 8).
The total costs of drug treatment for HCV are rising each year due to the introduction of new therapies, and in 2013 amounted to approximately 179 million PLN (based on the official report on activities of NHF in 4th quarter 2013 - concluded official data from contracts with hospital valued at 138.5 million). According to Polish National Health Fund data from 2013, 7 111 patients received at least one dose of drugs, sometimes not the full course) within drug program. With the estimated number of 231 thousand infected patient in Poland, this currently equates to a little more than 3% of the possible infected people. In the same year, costs of hospitalizations within diagnosis-related groups system (DRG) equalled 38 million PLN. The estimated cost of hepatitis C liver transplantations (caused by hepatitis C) is approximately 18 million PLN/year in Poland.
Loss productivity arise from generated by absenteeism and presenteeism. The absenteeism rate is the proportion of absence in time during which the respondent is supposed to work – premature death, sick leave, disability, holidays, family care. The presenteeism rate is the degree to which the health problem affected the respondent’s productivity while working, subjectively estimated by the respondent on a scale from 0 to 10 measured for example by WPAI (Work Productivity and Activity Impairment Questionnaire) questionnaire. It can be interpreted as the percent of work not done due to studied condition. The overall work impairment is the sum of work time lost by the respondent due to absenteeism and presenteeism (it is the sum of presenteeism rate multiplied by time spent at work and absenteeism rate). Total annual cost of loss of productivity is approximately 584.8 mln PLN – premature death 53.6 mln PLN, disability – 147.7 mln PLN (data from ZUS together for hepatitis C and B), sick leave – 20.9 mln PLN and maximum estimation of presenteeism for chronic C – 362.5 mln PLN.
Conclusion
The epidemiological data in Poland suggests that 3 thousand out of an estimated 231 thousand patients are currently being treated for hepatitis C infected patients. This translates into an estimated 20-40 thousand people suffering from cirrhosis of the liver during the next 20 years and 4 to 8 thousand patients needing treatment for hepatocellular carcinoma during the next 30 years if these patients remain untreated. For many patients, the only possible treatment will be liver transplantation. However, hepatitis C could now be treated very effectively. Early drug therapy averts serious health consequences for patients as well as reduces the chances of them transmitting the disease to others with cure rates higher than 95% with new therapies. It is recognised that this will appreciably add to drug costs in the early years; however the increased expenditure would be balanced against reduced costs of treating complications in the later years [46]. This could include the potential for discounted prices building on the experiences in other countries negotiated as part of risk sharing schemes for reimbursement [46]. Alongside this, there needs to be co-ordinated preventative activities and changes in treatment in Poland to reduce future infection rates as currently in Poland there are only a few screening activities (also in local governmental programs) and no strategic proposition of shaping the complex approach. If instigated, these could be as effective as actions taken to avoid HBV or HIV infections.
In view of the lack of any hepatitis C vaccination, we believe it is essential for the various national and regional authorities in Poland to take actions to stop the spread of the virus. These activities could be organized into: 1. education and promotion of behaviours that reduce the risk of further infections 2. providing national screening of high risk groups. 3. providing wider access to HCV tests, allowing early detection and diagnosis of the greatest number of people infected in local level. Such actions combined with the availability of effective and safe antiviral therapy could be as efficient as vaccination against hepatitis C, which, as previously pointed out, is non-existent at present. Preventive measures and treatment at early stages would also reduce the costs for the NHF of treating a significant number of patients with serious complications of hepatitis C including cirrhosis and hepatocellular carcinoma. In conclusion, we urge the various authorities in Poland to take prevention and management of HCV seriously, building on ongoing effective strategies for HBV and HIV.
- Maasoumy B., Wedemeyer H. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol. 2012; 26: 401–412
- Madaliński K., Flisiak R., Halota W. et al. Report prepared for The World Hepatitis Day - 28th July 2013; Available from: http://www.pzh.gov.pl/page/fileadmin/user_upload/aktualnosci/Report%20for%20Hepatitis%20Day%20WHO.pdf
- Madaliński K., Aktualny algorytm diagnostyki HCV; Available from: http://www.pzh.gov.pl/page/fileadmin/user_upload/SPPW/Konferencja_inauguruj%B9ca_11.10.2012/Prezentacje/P2.ppt
- Gajewski P. Interna Szczeklika - mały podręcznik 2014/2015; Available from: http://www.mp.pl/interna/
- WHO. The 63rd World Health Assemby. Resolutions and Decisions Annexes; Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA63-REC1/WHA63_REC1-en.pdf
- European Parliament. Written Declaration submitted under Rule 123 of the Rules of Procedure on Hepatitis B and C; Available from: http://www.europarl.europa.eu/sides/getDoc.do?type=WDECL&reference=P7-DCL-2013-0023&language=EN&format=PDF
- WHO. Viral hepatitis. Report by the Secretariat. A63/15; Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_15-en.pdf
- Ly KN., Xing J., Klevens RM. et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156: 271–278
- Mühlberger N., Schwarzer R., Lettmeier B. et al. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health, 2009; 9: 34
- ECDC. Annual Epidemiological Report: Reporting on 2011 surveillance data and 2012 epidemic intelligence data; Available from: http://www.ecdc.europa.eu/en/publications/Publications/annual-epidemiological-report-2013.pdf
- Bogucki M. Diagnostyka i terapia przewlekłego wirusowego zapalenia wątroby typu C (wirusem HCV) w Polsce: raport - rekomendacje 2013-2014; Instytut Ochrony Zdrowia; Available from: http://www.gwiazdanadziei.pl/download/raport_komisji_zdrowia.pdf
- Zieliński A. Wirusowe zapalenie wątroby typu B i C w Polsce w latach 1993-2011; Available from: http://www.pzh.gov.pl/page/fileadmin/user_upload/SPPW/Konferencja_inaugurujaca/prezentacje/Prezentacja%20-%20HCV%20-%20projekty.pptx;
- TNS OBOP. Wiedza na temat wirusowego zapalenia wątroby. Raport z badania. Available from: http://www.gwiazdanadziei.pl/download/raport_wiedza_na_temat_wirusowego_zapalenia_watroby_tns_.pdf
- WHO. Guidelines for the screening, care and treatment of persons with hepatitis C infection; Available from: http://apps.who.int/iris/bitstream/10665/111747/1/9789241548755_eng.pdf?ua=1&ua=1
- Esteban JI., Sauleda S., Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008; 48:148–162
- Godzik P., Kołakowska A., Madaliński K. et al. Prevalence of anti-HCV antibodies among adults in Poland-results of cross-sectional study in general population. Przegla̧d Epidemiol. 2012; 66: 575–580
- Flisiak R., Halota W., Horban A. et al. Prevalence and risk factors of HCV infection in Poland. Eur J Gastroenterol Hepatol. 2011; 23: 1213–1217
- Prometeusze. Information about hepatitis B and hepatitis C; Available from: http://www.prometeusze.pl/19V/Informator_WZW.pdf
- Statement of the President of the National Health Fund.; Available from: http://www.nfz.gov.pl/new/index.php?katnr=0&dzialnr=2&artnr=2910&b=1&szukana=wirusowe%20%2821.2.2013%29; [Accessed:17.10.2007]
- Godala M., Szatko F. Zgłaszalność chorób zakaźnych. Cz. I. Ocena świadomości lekarzy dotycząca zgłaszania chorób zakaźnych do inspekcji sanitarnej. Probl Hig Epidemiol. 2010; 91: 198–205
- NIPH - NIH. Infectious diseases and poisonings in Poland (annual reports); Available from: http://www.pzh.gov.pl/oldpage/epimeld/index_a.html
- ECDC. Surveillance and prevention of hepatitis B and C in Europe. European Centre for Disease Prevention and Control; 2010. Available from: http://ecdc.europa.eu/en/publications/Publications/101012_TER_HepBandC_survey.pdf
- Smith DB., Bukh J., Kuiken C. et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatol Baltim Md. 2014; 59: 318–327
- WHO. Hepatitis C. Global Alert and Responsis; Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index2.html#HCV
- Panasiuk A., Flisiak R., Mozer-Lisewska I. et al. Distribution of HCV genotypes in Poland. Przegla̧d Epidemiol. 2013; 67:11–16, 99–103
- Cieśla A., Mach T. Chronic viral hepatitis – current epidemiological, clinical and therapeutic challenge. Przegląd Gastroenterol. 2007; 2: 69–73
- CDC. Hepatitis C Information For the Health Professional; Available from: http://www.cdc.gov/hepatitis/HCV/index.htm
- EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2014; 60: 392–420
- Chen SL., Morgan TR. The Natural History of Hepatitis C Virus (HCV) Infection. Int J Med Sci. 2006; 3: 47–52
- Thein H-H., Yi Q., Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatol Baltim Md. 2008; 48: 418–431
- Juszczyk J., Baka-Cwierz B., Beniowski M. et al. Pegylated interferon-alfa 2a with ribavirin in chronic viral hepatitis C (final report). Przegla̧d Epidemiol. 2005; 59: 651–660
- Juszczyk J., Białkowska J., Bolewska B. Pegylowany interferon alfa-2b i rybawiryna w leczeniu przewlekłego wirusowego zapalenia wątroby typu C. Pol Merkuriusz Lek. 2004; XVI: 353
- Kołakowska-Rządzka A., Berak H., Wasilewski M. Relevance between fibrosis and response to treatment with peginterferon alfa2a vs alfa2b with ribavarin in chronic hepatitis C genotype 3 patients. Randomized open label study. Hepatol Baltim Md. 2008; 48: 878A
- Guido M., Mangia A., Faa G., et al. Chronic viral hepatitis: the histology report. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2011; 43 Suppl 4: S331–S343
- Hatzakis A., Van Damme P., Alcorn K, et al. The state of hepatitis B and C in the Mediterranean and Balkan countries: report from a summit conference. J Viral Hepat. 2013; 20 Suppl 2:1–20
- El-Serag HB., Kramer JR., Chen GJ. et al. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011; 60: 992–997
- Ollivier I., Dauvois B., Guittet L. et al. Survival improvement in Child-Pugh C cirrhotic patients with hepatocellular carcinoma diagnosed during 1990-2002. Gastroentérologie Clin Biol. 2010; 34: 288–296
- Borie F., Bouvier A-M., Herrero A. et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol. 2008; 98: 505–509
- Małkowski P., Czerwiński J., Pacholczyk M. et al. Current status of liver transplantation. Przegla̧d Epidemiol. 2005; 59: 559–566
- Małkowski P., Czerwiński J., Wasiak D. et al. Liver transplantation in Poland in comparison to European results. Med Sci Rev - Hepatol. 2007; 7: 3–8
- Mukherjee S., Sorrell MF. Controversies in liver transplantation for hepatitis C. Gastroenterology. 2008; 134: 1777–1788
- Poltransplant; Available from: http://www.poltransplant.org.pl/biuletyny.html
- Wawrzynowicz-Syczewska M. Przewlekłe wirusowe zapalenie wątroby typu C jako wskazanie do retransplantacji wątroby. Med Sci Rev - Hepatol. 2011; 11: 57–59
- Pawłowska J., Teisseyre M., Jankowska I. et al. Preliminary results and complications of HCV treatment after liver transplantation. Przegla̧d Epidemiol. 2006; 60: 677–683
- Liang TJ., Ghany MG. Current and Future Therapies for Hepatitis C Virus Infection. N Engl J Med. 2013; 368: 1907–1917
- Brennan T., Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014; 312: 593–594
- Halota W., Flisiak R., Boroń-Kaczmarska A. et al. Standardy leczenia wirusowych zapaleń wątroby typu C. Rekomendacje Polskiej Grupy Ekspertów HCV. Przegląd Epidemiol. 2012; 66: 83–88
- EMA. Product Characteristics - Rebetol (ribavirin); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000246/WC500048210.pdf
- EMA. Product Characteristics - PegIntron (peginterferon alfa-2b); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000280/WC500039388.pdf
- Jiménez-Sousa MA., Fernández-Rodríguez A., Guzmán-Fulgencio M. et al. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013; 11: 6
- Khuroo MS., Khuroo MS., Dahab ST. Meta-analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Aliment Pharmacol Ther. 2004; 20: 931–938
- Aljumah AA., Murad MH. Pegylated versus standard interferon plus ribavirin in chronic hepatitis C genotype 4: A systematic review and meta-analysis. Hepatol Res Off J Jpn Soc Hepatol. 2013; 43: 1255–1263
- Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006; 55: 1350–1359
- EMA. Product Characteristics - Victrelis (boceprevir); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002332/WC500109786.pdf
- EMA. Product Characteristics - Incivo (telaprevir); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002313/WC500115529.pdf
- EMA. Product Characteristics - Sovaldi (sofosbuvir); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002798/WC500160597.pdf
- EMA. Product Characteristics - Olysio (simeprevir); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_ _Product_Information/hu [Internet]. man/002777/WC500167867.pdf
- EMA. Product Characteristics - Daklinza (daclatasvir); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003768/WC500172848.pdf
- EMA. Product Characteristics - Harvoni (sofosbuvir/ledipasvir); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003850/WC500177995.pdf
- European Commission Grants Marketing Authorizations for AbbVie’s VIEKIRAX® (ombitasvir/paritaprevir/ritonavir tablets) + EXVIERA® (dasabuvir tablets) for the Treatment of Chronic Hepatitis C. Available from: http://www.prnewswire.com/news-releases/european-commission-grants-marketing-authorizations-for-abbvies-viekirax-ombitasvirparitaprevirritonavir-tablets--exviera-dasabuvir-tablets-for-the-treatment-of-chronic-hepatitis-c-300021695.html; [Accessed: 2015]
- Park C., Jiang S., Lawson KA. Efficacy and safety of telaprevir and boceprevir in patients with hepatitis C genotype 1: a meta-analysis. J Clin Pharm Ther. 2014;.39:.14–24
- AASLD. Recommendations for Testing, Managing, and Treating Hepatitis C. Available from: http://www.hcvguidelines.org/sites/default/files/full_report.pdf
- EASL Recommendations on Treatment of Hepatitis C 2014. Available from: http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf
- Jaroszewicz J., Flisiak R., Dusheiko G. A pill for HCV – myth or foreseeable future? Liver Int. 2014; 34: 6–11
- Flisiak R., Jaroszewicz J., Parfieniuk-Kowerda A. Emerging treatments for hepatitis C. Expert Opin Emerg Drugs. 2013; 18: 461–475
- Kowdley KV., Gordon SC., Reddy KR. et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. N Engl J Med. 2014;.370:.1879–1888
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2011; 17: 107–115
- Rosińska M., Parda N., Stepień M. Hepatitis C in Poland in 2011. Przegla̧d Epidemiol. 2013;.67:.247–251, 353–356