Generic substitution in the view of pharmaceutical scientists from Polish medical universities
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Generic substitution is a commonly observed phenomenon in pharmacy. It is promoted as part of the state drug policy and leads to a reduction in expenditure on drugs, facilitates the purchase of medicines by patients and improves compliance. So far researchers have focused on the evaluation of substitution by pharmacists, doctors or patients. The present study investigates the opinion of Pharmaceutical Faculties employees. The respondents acknowledge the importance of generic substitution; nevertheless they do not have a clear opinion on the effectiveness and safety of generic products. The respondents’ experience may have had a significant impact their opinion, especially of those working in the community pharmacies. The studies is part of a broader framework of the national debate on generic substitution. Undoubtedly the approach to generic substitution is one of the contemporary challenges of the pharmaceutical law.
Introduction
Generic substitution is the term applied to the substitution of a prescribed branded drug with a different form of the same active substance [1]. This method is used for the optimization of the costs of medicines. The retrenchment on substitution may be used to reimburse innovative molecules. Such conclusion inspired the legislators who passed a new Polish law in 2012 [1,2]. According to the legislature, pharmacists are obliged to inform the patient about the possibility of purchasing a cheaper and refundable substitute of a given drug. To promote generic substitution, The Office for Registration of Medicinal Products, Medical Devices and Biocidal Products was involved, and materials have been sent to patients, pharmacists and physicians [3]. The drawn opinions have been widely discussed issues of pharmacy substitution [4]. The pharmaceutical law provided no possibility of refusing to convert the drug to a cheaper equivalent by the pharmacist, based only on their pharmaceutical knowledge. This is an important difference to the preferred solutions in Western Europe and a challenge for the unification of the Polish legislation with the practice in the European Union [5].
Materials and Methods
The aim of the project, was to gain insight into the opinion of the Pharmaceutical Faculties scientists in Poland, on the economic aspects of generic substitution. The research sample was randomly selected. A questionnaire consisting of 11 cloze and 1 open questions was created and tested for its face and content validity. The Likert Scale and modifications was used. Only non-projective questions were used. A pilot study on the questionnaire was carried out on 10 respondents. The characteristics of the respondents were created based on the responses to questions about the academic degree or title, and any work experience in a pharmacy. The survey was conducted electronically among academics and didactic teachers. For each of the 10 Polish pharmaceutical departments 50 requests were sent by email. The ethical approval was not required for this study.
Results
In total, 116 employees of Pharmaceutical Faculties, completed the questionnaire. The study group was diverse in terms of obtained professional degree: 37% of the respondents obtained the professional title of Master, 39% of the doctoral degree, 14% with the post-doctoral title (Assistant Professor) and 6% of professors.
Among academics who participated in the study, 30% are actively practicing as a pharmacist in the community pharmacies.
Table 1. The structure of respondents by degree
| 1 | 37,00% | Master |
| 2 | 39,00% | PhD |
| 3 | 14,00% | Assistant Professors |
| 4 | 6,00% | Professors |
| 5 | 4,00% | No information |
The respondents did not have an unanimous opinion on the effectiveness of generic drugs.In the opinion of almost 50% of the respondents there are differences in efficacy between the innovative drugs and generic products. However, almost the same number of respondents, indicated a lack of such differences.
It is worth emphasizing that 61% of respondents did not associate additional therapeutic risks with the conversion of the original on the generic. The responses may be related to the professional experience of the respondents. Perhaps patients in pharmacies indicate the differences in the efficacy of drugs in the absence of an increased number of adverse events.
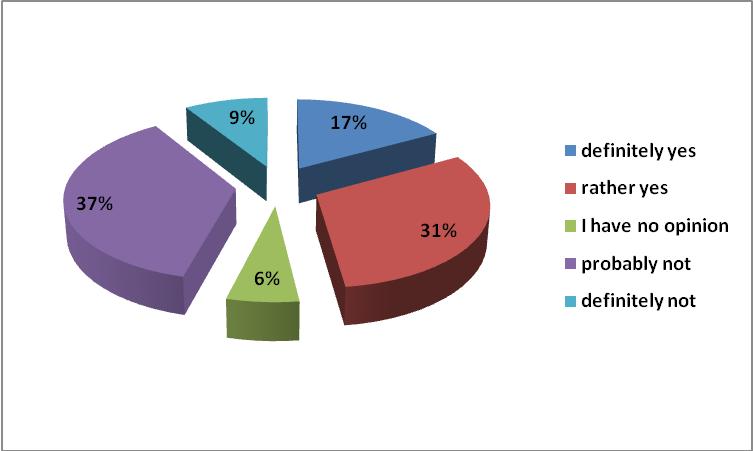
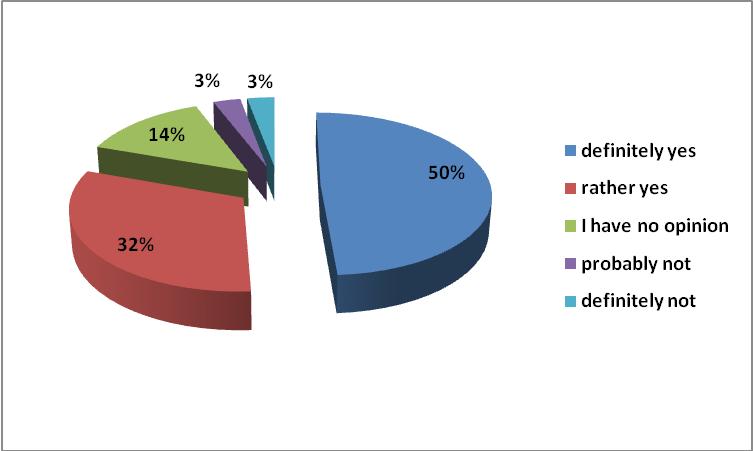
According to 72% of respondents, generic substitution should be promoted as a way of rationalizing the expenditure on drugs; and according to 69% as a way of reducing household spending on drugs. The level of co-payments for drugs in Poland should be considered as too high. Patients, especially the elderly and chronically ailing, may have difficulty in obtaining the necessary medicines. This may have an impact on compliance, which in turn could lead to an increase in health care spending. Commonly known mechanisms should be able to refer patients to hospitals instead of outpatient treatment [6].
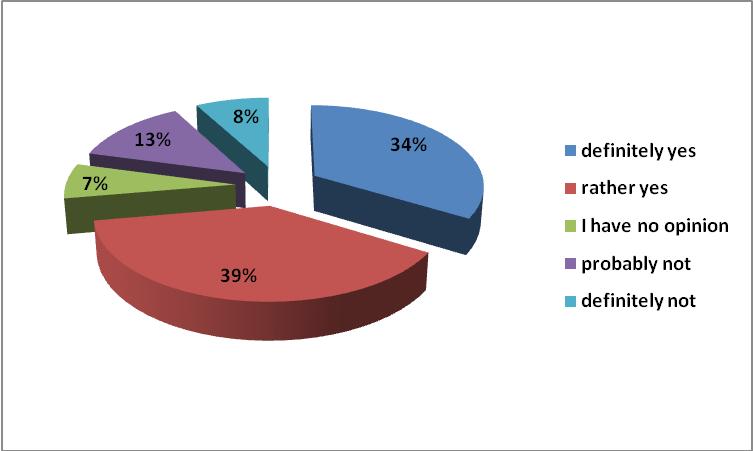
An extremely important issue of modern pharmacology is monitoring adverse reactions to drugs. The Polish pharmaceutical law refers to the definition of the adverse effects of the medicinal product - not the active pharmaceutical ingredient. This was reflected in the survey results. 71% of respondents emphasized that the cost of the delivered equivalent should be monitored. The National Health Fund also based on data relating to the possible increased incidence of adverse events.
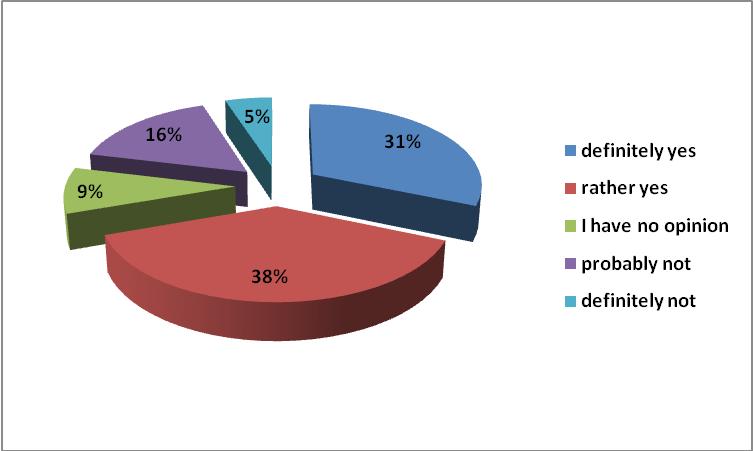
In the opinion of 82% of respondents, the financial surplus obtained on generic substitution, should be used to expand the list of reimbursed drugs. It is worth noting that the introduction of new rules for reimbursement of medicines has led to the introduction of new reimbursed, innovative molecules. However, access to the innovative substances for Polish patients, should be viewed as difficult, particularly when we compare the situation in Poland and the western countries of the European Union.
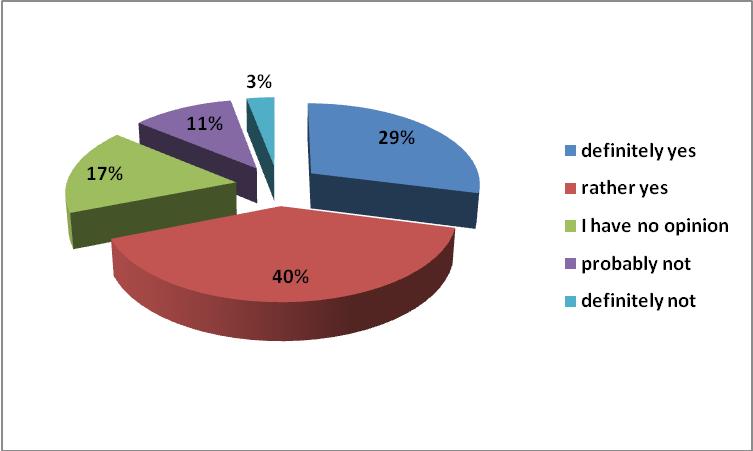
According to 69% of respondents, generics have to be an important component of hospital formularies. Formularies are now a commonly encountered form of rationalization of expenditures for drugs in hospitals. The adopted drugs available in inpatient care should be divided into 3 categories: A - to be applied by any physician, B - requiring the consent of the chief, C - only with the consent of the Director of Medical devices. This distinction has been widely accepted.
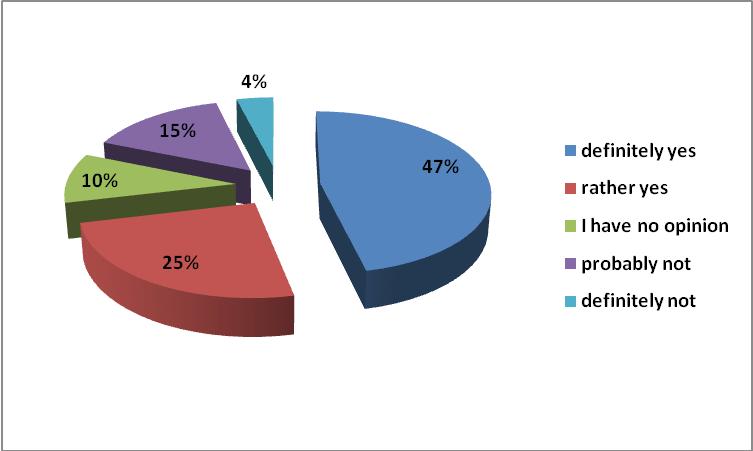
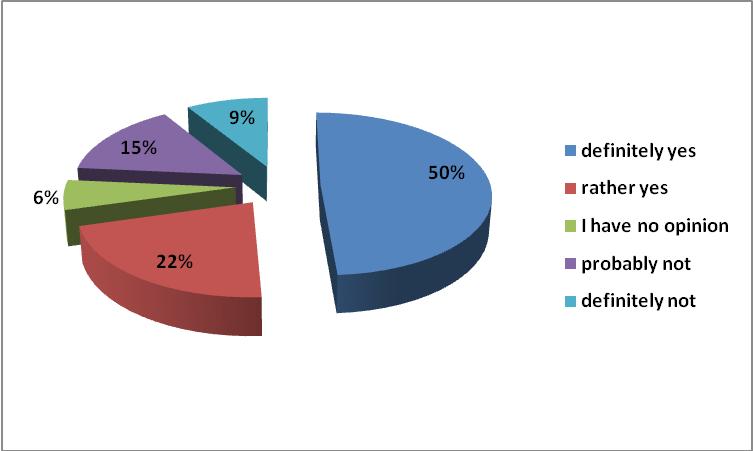
72% of respondents believe that the pharmacist should be able to refuse to convert the drug to a cheaper generic equivalent if they consider it appropriate in view of the available medical and pharmaceutical knowledge. Nowadays, only physicians have complete freedom in the process of prescribing. Pharmacists were obliged by law to inform patients about the possibility of purchasing a cheaper substitute drug. Nevertheless they cannot legally refer to their pharmaceutical knowledge, even if the used drug has a narrow therapeutic index and conversion should not take place. In Europe, various countries have prepared pharmacy substitution standards for determining such issues. The legislature did not provide for the possibility of introducing the Good Pharmacy Practice: a document containing a description of the procedures to facilitate the use of the pharmacists’ knowledge for the benefit of patients.
Figure 7. Should the pharmacist be able to refuse to convert the drug to a cheaper generic equivalent if they considers it appropriate in view of the available medical and pharmaceutical knowledge?
Discussion
Substitution is a relatively well-explored phenomenon in the pharmaceutical market. One of the global challenges facing pharmacology is the unification of the law. The process of harmonization is ongoing within the countries of the European Union. Different countries have different regulations on legal issues related to generic medicines and the process of substitution. Undoubtedly the approach to generic substitution is one of the contemporary challenges of the pharmaceutical law [7]. There have also been considerable debates about bioequivalence and generic substitution of certain critical care drugs. Substitution is an important phenomenon, especially for patients suffering from chronic illnesses. One study investigated the attitudes of patients suffering from epilepsy to substitution. More than 70% of the patients were concerned with the effectiveness of generic antiepileptic drugs and 68% of the patients were not comfortable receiving generics to treat epilepsy. About 87% of the patients thought that their antiepileptic drug should only be substituted by a generic with their consent, and 64% of the patients believed that substitution should only take place with the consent of their doctor [8]. Other studies came toquite similar conclusions. Substitution may result in a reduction in compliance. Personal interviews with 174 Norwegian patients (50–80 years) who had had their brand-name antihypertensive drug generically substituted, were conducted using a semi-structured questionnaire. The study shows that generic substitution can be an additional factor in poor drug adherence in hypertensive patients and contributes to concerns and confusion among the patients [9]. Similar conclusions were also drawn by researchers from Australia. For patients, the price is not the most important feature. Ill people appear to have a strong commitment to a specific medicine product. The findings show that customers with poor awareness of generic prescription medicine, when offered a substitute, were likely to become confused and suspicious. Pharmacists, on the other hand, experienced frustration due to the mistrust and annoyance their customers displayed[10]. The differences in efficacy between the original and generic drugs are confirmed by scientific research. It seems to be a reasonable argument that the payer should consider the pharmacotherapy cost based side effects. Of 671 patients treated with branded lamotrigine, 187 patients (27.9%) switched to a generic, and 51 of these patients (27.5%) switched back to the branded medication. The switch to generic lamotrigine was significantly associated with an increased number of physician visits and hospitalizations [11]. One of the main arguments for substitution is saving measures; however, empirical research shows a different trend. A careful analysis demonstrates the only a part of the substitution process leads to cost saving policies. Finland proves to be a good example of the above mentioned issues. The study analysed the economic assessment of substitution focusing on the impact of reference pricing and extension of generic substitution on the daily cost of antipsychotic drugs in Finland during the first year after its launch. Furthermore, the additional impact of reference pricing on prior implemented generic substitution is assessed. Reference pricing and the extension of generic substitution produced substantial savings on antipsychotic medication costs during the first year after its launch, but the intensity of the impact differed between active substances. Furthermore, results suggest that the additional cost savings from reference pricing after prior implemented generic substitution, are comparatively low [12].
Conclusions
According to the respondents:
1) Substitution is the way to rationalize expenditure on drugs.
2) There are differences in efficacy between the drugs and generic companies.
3) The use of generics is not associated with an increased risk of side effects.
4) The costs relating to the possible increased incidence of adverse events deriving from the use of the counterpart should be monitored by the National Health Fund.
5) The financial surplus obtained on generic substitution should be used to expand the list of reimbursed drugs.
6) Generic substitution should be promoted among physicians as a way of reducing the spendings on drugs.
7) Generics should be an important component of hospital formularies.
8) The pharmacist should be able to refuse the drug exchange for cheaper generic equivalent if they consider it appropriate in view of the available medical and pharmaceutical knowledge.
Experience may have had a significant impact on the opinions of respondents, especially those working in the community pharmacies. Undoubtedly, the approach to generic substitution is one of the contemporary challenges of the pharmaceutical law. The studies represent another part in the national debate on generic substitution.
- Posner J., Griffin J., Br J Clin., Generic Substitution, Pharmacol. 2011; 72(5): 321-322
- Act on the Reimbursement of Medicines, Foodstuffs Intended for Particular Nutritional Uses and Medical Devices, Poland, 2012
- The Office for Registration of Medicinal Products, Medical Devices and Biocidal Products. Available from: http://en.urpl.gov.pl; [Accessed: 14.12.2014]
- Hakonsen H. et all, Generic substitution: an additional challenge for adherence in hypertensive patients?. Curr Med Res Opin. 2009, 25(10): 2515 – 2521
- Durdeon M., Hughes D., Generic and therapeutic substitutions in the UK: are they a good thing?. Br J Clin Pharmacol 2010, 70(3): 335–341
- Wpływ ustawy o refundacji leków na dostęp pacjentów do farmakoterapii (..) Ocena skutków regulacji. Raport Infarma, Warszawa 2014
- Handoo S., Arora W., Khera D., A comprehensive study on regulatory requirements for development and filing of generic drugs globally. Int J Pharm Investig, 2012; 2(3): 99 – 104
- Stupans I., McKinnon R., Generic substitution in the treatment of epilepsy: patient attitudes and perceptions. Epilepsy Behav. 2013; 26(1): 64-66
- Hakonsen H., Eilertsen M. et all, Generic substitution: an additional challenge for adherence in hypertensive patients?. Curr Med Res Opin. 2009; 25(10): 2515 – 2521
- Gill L., Helkkula A., Cobelli N., White L., How do customers and pharmacists experience generic substitution?, International Journal of Pharmaceutical and Healthcare Marketing 2010; 4(4): 375 – 395
- LeLourier J. et all, Clinical consequences of generic substitution of lamotrigine for patients with epilepsy, Contemporary Issues in Neurological Practice 2008; 2179 – 2186
- Koskinen H., Ahola E. et all, The impact of reference pricing and extension of generic substitution on the daily cost of antipsychotic medication in Finland. Health Economics Review 2014; 4(9)