Assessment of the financial barriers in access to cancer pain treatment with opioids in Polish settings
-
Copyright
© 2015 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Tomasz Faluta |
Wojskowy Instytut Medyczny |
|
Wojciech Giermaziak |
Main Medical Library |
Effective pain management and pain prevention is one of the fundamental obligations of doctors. The need to improve the management of pain is widely recognized in public debate in Poland.
Background: Identification and assessment of financial barriers in access to opioids in pain treatment of cancer patients in Poland.
Material and Methods: The main area of investigations was availability of opioids and cost of treatment for patients. The principles, conditions and procedures for reimbursement of opioids were recognized and analyzed from cancer patients point of view. All reimbursement lists announced by MoH were taken into account as well as data concerning reimbursed drug consumption and NHF spending’s.
Results: Polish cancer patients have access to subsequent reimbursed opioids: morphine, fentanyl, oxycodone, methadone, buprenorphine, tramadol, dihydrocodeine.
In the case of opioids limit groups are narrow and very precisely defined and very compartmentalized. In practical terms, almost all patients may have access to opioids with limit price without additional co-payment.
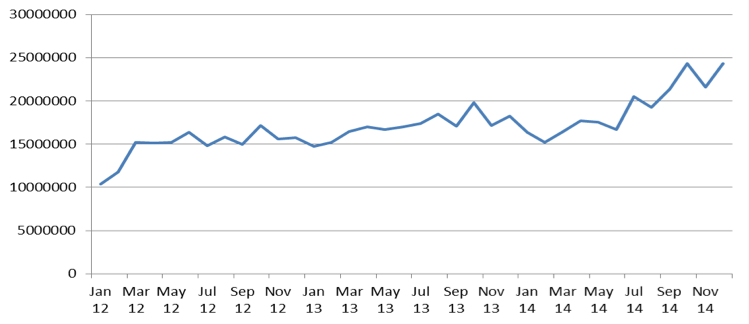
Expenditures on opioids reimbursement between 2012 and 2014 increased from PLN 178M to 231M. Consumption of opioids increased faster than reimbursement expenditures between 2012 and 2014 because growth of consumption between 2012 – 2013 and 2013-2014 accounted for 17,7% and 23,4% respectively. Significant price decrease of opioids leaded to decrease of average patients co-payment for pack of opioids from PLN 9,63 to PLN 6,10.
Conclusions: Financial barriers in access to cancer pain treatment with opioids in Poland seems to be very limited.
Introduction
Effective pain management and pain prevention is one of the fundamental obligations of doctors. The need to improve the management of pain is widely recognized by many stakeholders as a hot issue in public debate in Poland. The Standing Committee of the Supreme Medical Court addressed the appeal to doctors and dentists to ensure the effective pain management to all patients. Improvement of pain management in cancer patients seems to be most important need. Information and education program called “Pain free cancer” (“Rak wolny od bólu”) is conducted by Patients Organizations in cooperation with Ministry of Health. According to experts from Fight against Pain (NGO) one of the barriers in pain treatment is fear of opioids as a results of ignorance and of the rules of using this group of medicines [1]. Many patients feel fear to take opioids. They are afraid to become drug addicted or even to be suspected. One of the main concerns of physicians when prescribing opioids is that the daily doses used for pain relief may predispose patients to drug dependence [2-4]. In 1986 the World Health Organization (WHO) presented the analgesic ladder as a framework that physicians could use when developing treatment plans for cancer pain. If cancer pain occurs, there should be prompt oral administration of drugs in the following order: nonopioids (aspirin and paracetamol); then, as necessary, mild opioids (codeine); then strong opioids such as morphine, until the patient is free of pain. To maintain freedom from pain, drugs should be given “by the clock”, that is every 3-6 hours, rather than “on demand” In the case of cancer pain in children, WHO recommends a two step ladder[5-7].
Background
Identification and assessment of financial barriers in access to opioids in pain treatment of cancer patients in Poland.
Material and Methods
The main area of investigations was availability of opioids and cost of treatment for patients. The principles, conditions and procedures for reimbursement of opioids were recognized and analyzed from cancer patients point of view. Time horizon for observation was limited to years 2012-2014 because current reimbursement regulations were introduced in 2011 [8]. All reimbursement lists announced by the minister responsible for health matters (MoH) [9] were taken into account as well as data concerning reimbursed drug consumption and National Health Found (NHF) spending’s, published by the Drugs Management Department of National Health Found [10]. Opioids consumptions were converted to DDD (define daily dose) [11] because the purpose of the DDD system is to serve as a universal tool for drug utilization research in order to improve quality of drug use. DDD system allows to study over time trends in drug consumption without the complication of frequent changes in the reimbursement system.
Results
Assessment of the principle for opioids reimbursement in Poland
The reimbursement principles are regulated by Act of 12 May 2011 on the reimbursement of medicinal products, special purpose dietary supplements and medical devices [8].
Polish cancer patients have access to subsequent reimbursed opioids: morphine, fentanyl, oxycodone, methadone, buprenorphine, tramadol, dihydrocodeine. Drugs reimbursement for polish patients may be specified in four categories: drugs available in the pharmacy on prescription, drugs used in a regimen programme (drug programme), drugs used in chemotherapy and drug used within the other guaranteed procedures. All reimbursed opioids are drugs available in the pharmacy on prescription and administrative restrictions are limited to minimum in comparison to the rest kinds of reimbursement.
Reimbursed opioids are available to patients free of charge or for a lump sum fee 3,20 (~ € 0,8), but NHF reimbursed drugs costs only to the limit price. If price of drug is higher than the limit price, patient has to pay the difference.
The criteria to set limit groups are not defined precisely. According to Article 15.2 Act of reimbursement, a drug which has the same international name or different international names, but similar therapeutic effects and similar mechanism of action and qualifies for inclusion in a limit group has to meet the following criteria: the same indications or assignments, in which they are reimbursed; similar efficacy. This definition in many cases effects in creation of jumbo limit group e.g. all statins, all ACE-Is. In the case of opioids limit groups are narrow and very precisely defined and very compartmentalized. This means in practical terms that almost all patients may have access to opioids with limit price without additional co-payment. It looks as a patients friendly application of law. Overview of limit groups is showed below in Table 1.
Table 1. Reimbursement limit groups for opioids used in the treatment of cancer pain
| International name (INN) | Limit groups | Payment |
| Morphine | 149.1, Opioid pain killers –morphine orally administered - prolonged release form. | free of charge |
| Morphine | 149.2, Opioid pain killers –morphine oral administered - prolonged release form | free of charge |
| Morphine | 149.3, Opioid pain killers –morphine orally administered - normal release form. | free of charge |
| Oxycodone | 150.1, Opioid pain killers – oxycodone | lump sum fee |
| Dihydrocodeine | 150.2 Opioid pain killers – dihydrocodeine | free of charge |
| Methadone | 150.3, Opioid pain killers – methadone | lump sum fee |
| Oxycodone + Naloxone | 150.4, Opioid pain killers – oxycodone in combinations | free of charge |
| Fentanyl | 152.1, Opioid pain killers – buccal form | lump sum fee |
| Fentanyl | 152.2, Opioid pain killers – nasal form | lump sum fee |
| Buprenorphine | 152.3, Opioid pain killers – sublingual form | free of charge |
| Buprenorphine, | 152.4, Opioid pain killers – transdermal form | free of charge |
| Fentanyl | 152.4, Opioid pain killers – transdermal form | lump sum fee |
| Tramadol | 153.1, Opioid pain killers - tramadol – rectal form | free of charge |
| Tramadol | 153.2, Opioid pain killers - tramadol – parenteral form | free of charge |
| Tramadol | 153.3, Opioid pain killers - tramadol – oral form | free of charge |
| Tramadol + Paracetamol | 153.3, Opioid pain killers - tramadol – oral form | free of charge |
| Tramadol | 153.4, Opioid pain killers - tramadol – oral form – liquid | free of charge |
National Health Found reimbursement expenditures on opioids in 2012-2014
Expenditures on opioids reimbursement were important and growing part of National Health Found reimbursement budget. Between 2012 and 2014 reimbursement expenditures on opioids increased from PLN 178M to 231M. Increase of reimbursement expenditures on opioid seems to have a constant trend, because the growth between 2012 – 2013 and 2013 – 2014 accounted for 15,2% and 12,7% respectively. Constant growing trend seems to be confirmed on monthly chart of expenditures (Figure 1).
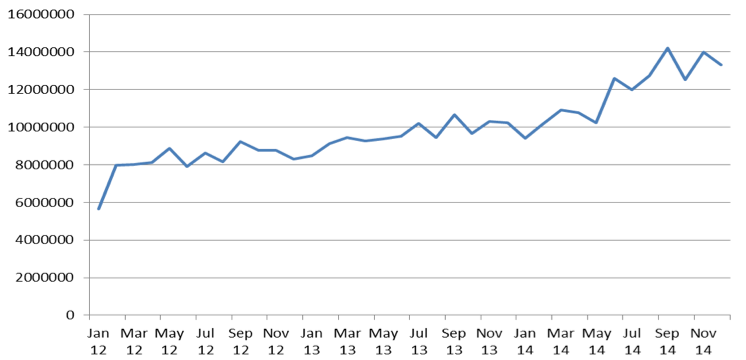
This growing trend of opioid consumption is also confirmed by the growth expressed in DDD on monthly basis (Figure 2).
Consumption of opioids increased faster than reimbursement expenditures between 2012 and 2014 because growth of consumption between 2012 – 2013 and 2013-2014 accounted for 17,7% and 23,4% respectively when increase of expenditures accounted for only 15,2% and 12,2 % respectively (Table 2).
Table 2. Comparison of the growth of budget expenditures on opioids and growth of opioids utilization by patients
| Year | 2012 | 2013 | 2014 |
| Number of DDD reimbursed by NHF | 98 380 209 | 115 832 417 | 142 952 655 |
| Growth | 17,7% | 23,4% | |
| Costs of reimbursement for NHF budget (PLN) | 178 154 144 | 205 310 655 | 231 398 054 |
| Growth | 15,2% | 12,7% |
This difference might be explained as a result of price decrease of opioids. During three years average price was decreased by 14,8% (Table 3).
Table 3. Results of comparison of prices decreases, limit price decreases and patients co-payment between 2012 and 2014
| Period | 2012 | 2013 | 2014 | 2012 - 2014 |
| Average price decrease | -4,36% | -6,55% | -1,38% | -14,80% |
| Minimal price decrease | -0,72% | -0,66% | 0,00% | -1,59% |
| Maximal price decrease | -32,07% | -19,57% | -17,11% | -64,31% |
| Average change of limit price | -0,20% | -8,86% | -0,51% | -9,62% |
| Minimal decrease of limit price | 37,74% | -0,66% | 0,00% | 14,34% |
| Maximal decrease of limit price | -22,23% | -20,31% | -4,28% | -33,02% |
| Average change of patients co-payment | -11,0% | 22,9% | -22,5% | -17,3% |
| Maximal increase of patients co-payment | 159,1% | 988,7% | 283,8% | 408,4% |
| Maximal decrease of patients co-payment | -100,0% | -45,3% | -100,0% | -100,0% |
Significant price decrease of opioids leaded to decrease of average patients co-payment for pack of opioids from PLN 9,63 to PLN 6,10. (Table 4).
Table 4. Comparison of changes in patients co-payment for opioids pack between 2012 and 2014
| Beginning of 2012 | Beginning of 2013 | Beginning of 2014 | End of 2014 | |
| Average patients co-payment (PLN) | 9,63 | 6,90 | 8,20 | 6,10 |
| Maximal patients co-payment (PLN) | 103,14 | 30,60 | 74,36 | 29,80 |
| Minimal patients co-payment (PLN) | 0,00 | 0,00 | 0,00 | 0,00 |
Conclusions
Financial barriers in access to cancer pain treatment with opioids in Poland seems to be very limited, especially when compared to other drugs. Reimbursement limit groups for opioids are narrow and very precisely defined. Almost all patients may have access to opioids with limit price without additional co-payment. Average patients co-payment to one pack of opioids during last three year wat decrease only to PLN 6,10 (~ € 1,5). Increase on opioid consumption from 98 million DDD to 142 million DDD in three years indicates on lack of significant financial barriers in access to cancer pain treatment with opioids.
- Ciałkowska-Rysz A., Dzierżanowski T., Podstawowe zasady farmakologii bólu u chorych na nowotwory i inne przewlekłe, postępujące, zagrażające życiu choroby. Medycyna Paliatywna 2014; 6(1): 1-6
- ACHELON Working Group, Current practices in cancer pain management in Asia: a survey of patients and phisiscians across 1o countries, Cancer Med. 20`15 Apr. doi:10.1002/cam4.471
- Brennan MJ., Stanos S., Strategies to optimize pain management with opioids while minimizing risk of abuse, PM R. 2010 June; 2(6):544-58
- Ahlbeck K., Opioids: a two-faced Janus, Curr Med Opin. 2011 Feb; 27(2):439-48
- World Health Organization. Traitement de la douleur cancéreuse. Geneva, Switz: World Health Organization; 1987
- Available from: http://www.who.int/cancer/palliative/painladder/en/
- Available from: http://whqlibdoc.who.int/publications/2012/9789241548120_Guidelines.pdf?ua=1
- Dz.U. 2011 nr 122 poz. 696.
- Available from: www.mz.gov.pl
- Available from: www.nfz.gov.pl
- Available from: http://www.whocc.no/atc_ddd_index/