The evolving landscape for Real World Evidence in Poland: physicians' perspective
-
Copyright
© 2015 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Real World Evidence is increasingly being demanded by national payers and HTA agencies to substantiate and validate outcomes from clinical trials. Yet, the access and quality of existing databases is often very limited, what creates hurdles to evaluate evidence in ‘normal’ healthcare setting. In Poland key stakeholders have not yet fully embraced RWE as an evidence source though they have growing need for more evidence to allocate scarce resources. The coming years are crucial for the shape and accessibility of RWE in Poland – however, the standpoint and the needs of the key stakeholders are not yet recognized. Therefore, an exploratory survey was undertaken to map current awareness and expectations of physicians related to practical outcomes data and to identify pharmaceutical industry’s role in RWE generation. Consideration of stakeholder needs seems to be the natural step in the beginning of preparation for real world evidence system in Poland. The analysis showed significant physicians interest in practical evidence and broad spectrum of possible actions that can be undertaken to improve formal use of RWE in Poland. The differences in priorities between specializations are a good indicator of the unmet needs in certain therapeutic areas. Broadening doctors’, payers’, insurers’ and service-providers’ knowledge on RWE significance, raising awareness of practical implementation and improving accessibility could be crucial for shaping the RWE landscape in Poland and, consequently, for improving patient treatment results.
Introduction
Due to the rapidly changing range of medicines and therapies, the healthcare system stakeholders are increasingly turning to practical evidence for decision support. As a result there is a growing need for access to data that could explain reasons for initiation, combination and sequencing of different treatment options in non-trial setting (i.e. outside the framework of clinical studies). The starting point in estimating the effectiveness of a medical treatment is to collect key information about the number of exposed patients, drug utilisation, and the actual patient outcomes. In Poland, this kind of information is rare. Both medical practitioners and decision-makers in the healthcare system, at the local and national level, are not given the tools with which they could effectively monitor the progress of therapy, or evaluate treatment outcomes in the long term. A rising need for new medical registers or structured databases is increasingly recognised, offering the opportunity to compare the effectiveness of existing therapeutic options in conditions more closely reflecting everyday medical practice.
Definitions, sources and usability
In professional literature, this type of healthcare data is termed Real World Evidence (RWE) or Real World Data (RWD). This is a general term describing various types of data sets accommodating information about epidemiology, effectiveness, safety, and costs of treatment, generated and analysed outside the framework of randomised controlled trials (RCTs) [1]. In other words, RWE delivers insights into patient outcomes in real-life setting, in healthcare conditions other than meticulously arranged study conditions.
|
Real World Evidence (RWE)– data from the actual medical practice. |
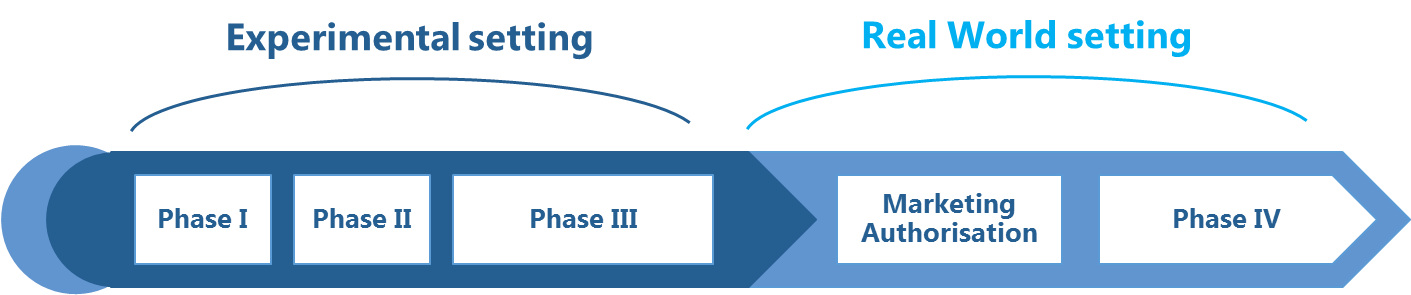
Clinical trials are an obligatory part of the process of granting a marketing authorisation for almost every new medicinal product. Clinical trials are carried out to confirm safety and efficacy of an investigational medicinal product and to define its therapeutic effect in a study population. Phase III studies – the most important phase of a clinical trial that translates into future clinical recommendations – are typically conducted in a highly selected group of patients who grant an informed consent to take part in the study, while the progress of the study and the follow-up period (lasting several months to several years) are described in much detail in the study protocol.
|
In experimental setting, the quantitative measures with which the study sample is described (study end points) and the progress of the study are closely monitored and controlled to be able to prove that changes in the end point values result directly (and solely) from exposure to the investigational medicinal product. Later on, a medicinal product authorised for marketing is used in a more general population of patients who can suffer from co-morbidities and other health problems, or use concomitant medications, while receiving medical care in a complex healthcare setting, determined for example by the structure of the healthcare system. |
Although they deliver best-quality scientific data, RCTs do not provide information about:
- treatment effectiveness in actual (everyday) medical practice, both in clinical and economic terms,
- effects of treatment on real-world, normal population instead of (selected) study population (i.e. where various inclusion and exclusion criteria apply to avoid enrolling patients with co-morbidities), or the prevalence of rare adverse effects, or drug-to-drug interactions;
- long-term data about correlations between safety and efficacy of therapy and real-world patient behaviour (e.g. patient compliance).
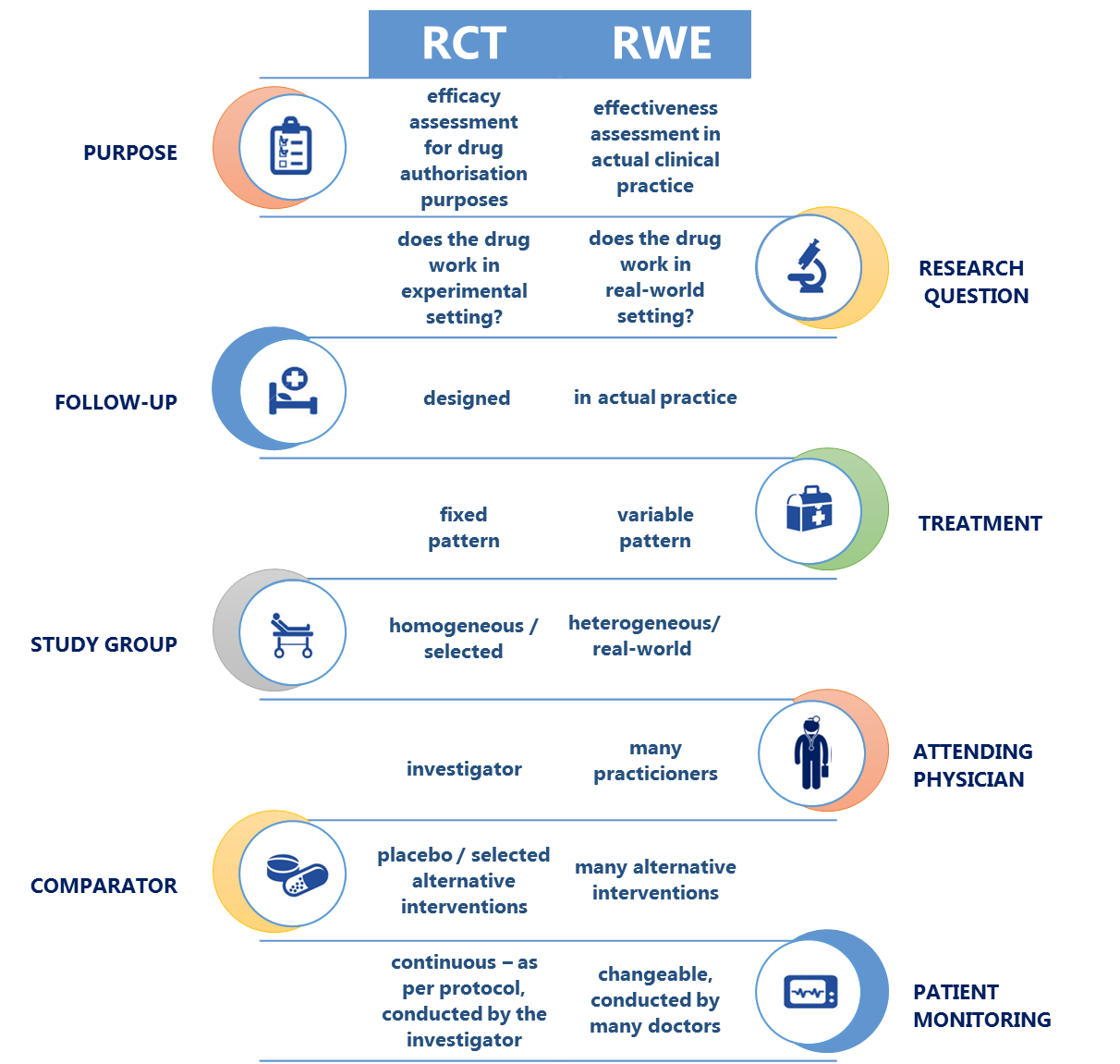
Unlike pre-marketing clinical studies investigating “efficacy”, RWE provides information about “effectiveness”. Based on tracing the 'real-world' medical history of patients dating many years back, data collected from a broader patient population, and evidence of real-life patient compliance, RWE is complementary to conventional data from RCTs, and as such it paints a wider picture of the methods used in preventing, diagnosing, and managing specific diseases, and of the long-term safety, effectiveness and costs of therapy. RWE appears to be an adequate response to the increasing demand of the healthcare system for more comprehensive evidence.
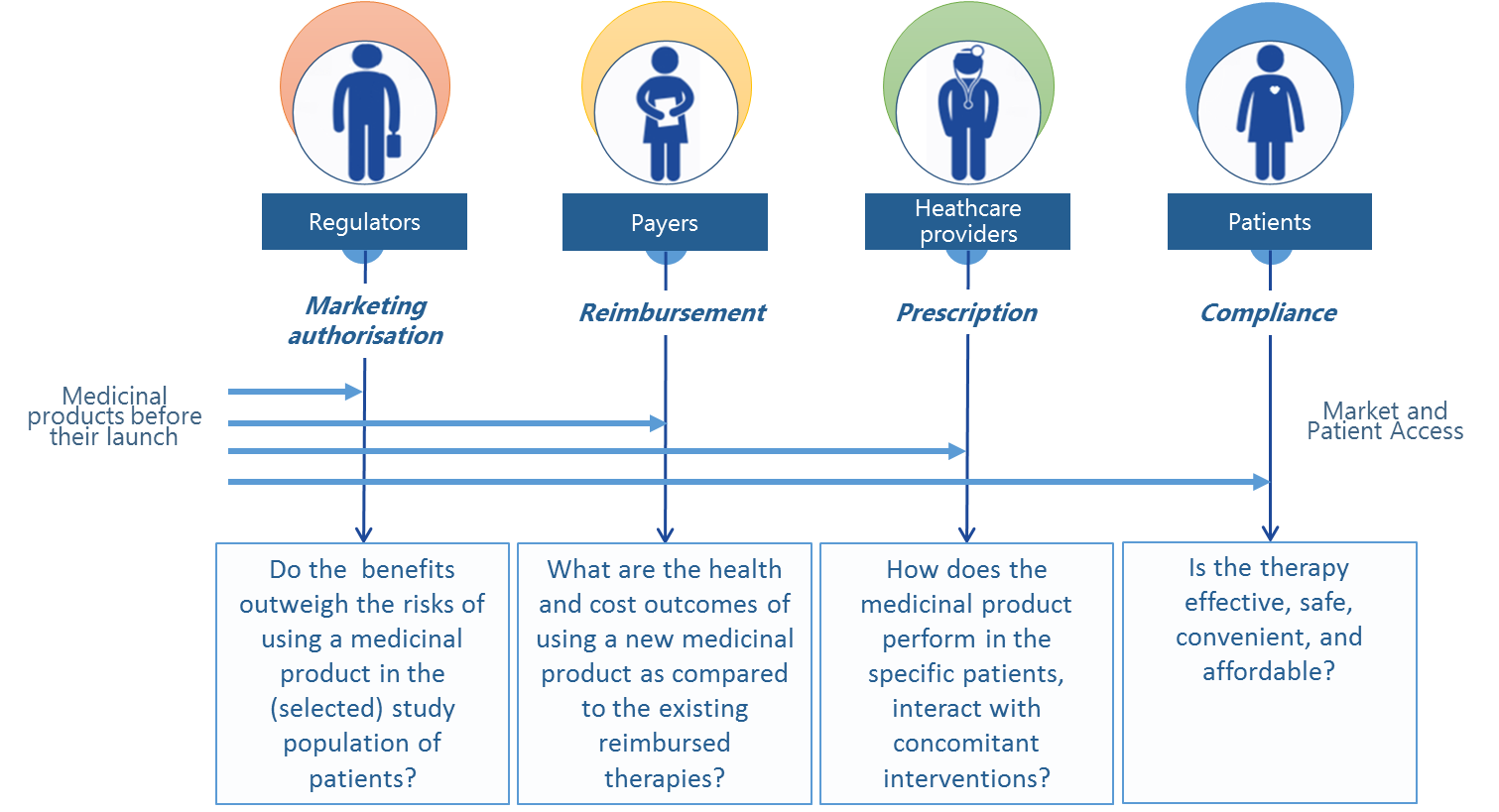
Apart from the scientific value it carries, information from medical registers that describe real-world treatment outcomes can serve as a basis for a system-wide economic and social assessment of medical technologies. Therefore in many countries across Europe, insurance institutions (payers), regulatory authorities, or agencies that evaluate the cost-effectiveness of medical technologies increasingly demand access to this type of information and knowledge – in addition to the outcomes of RCTs.
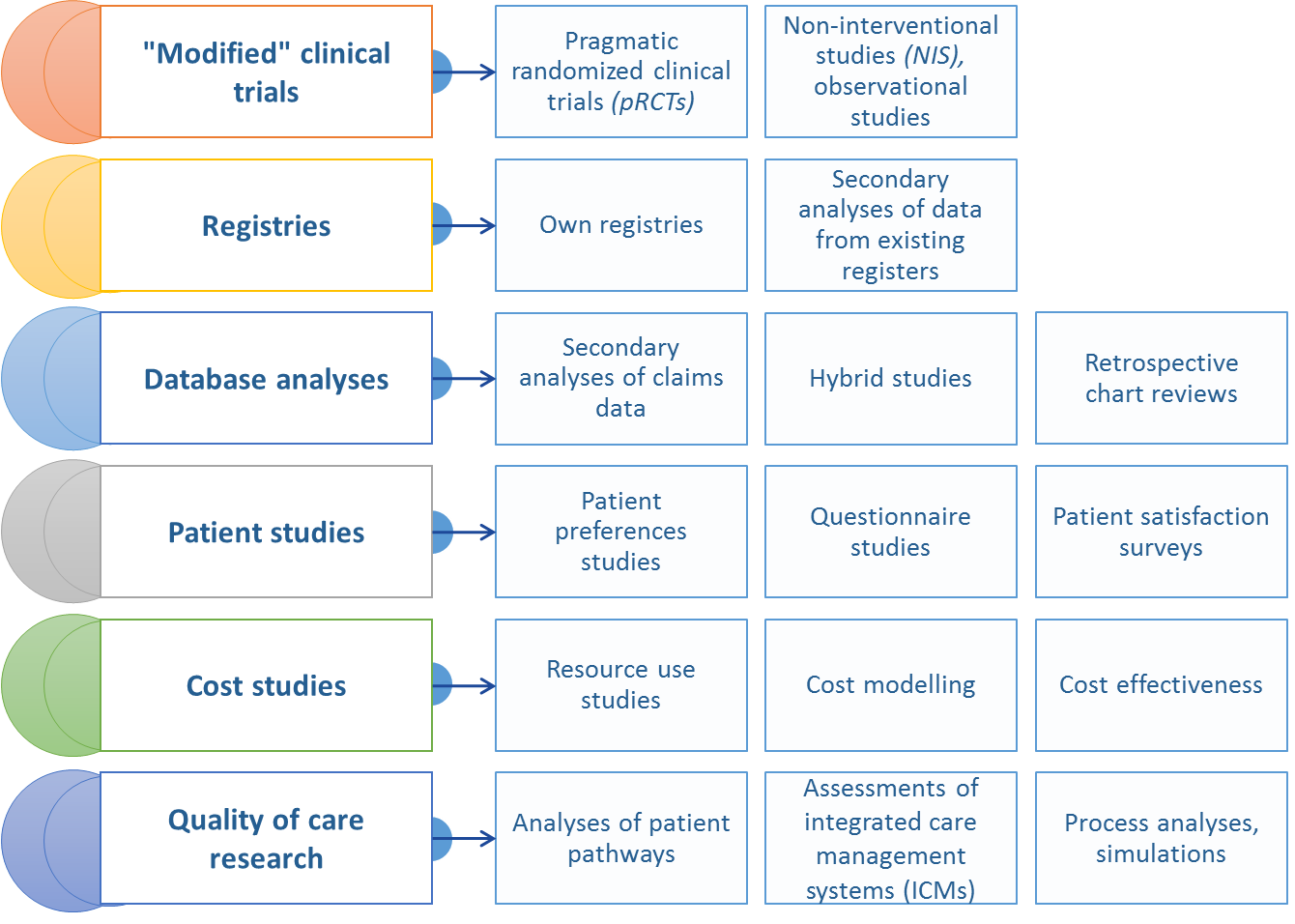
Real World Data can be derived from different types of registers (disease based, drug based), economic or social databases collected from medical practitioners or healthcare centres, patients, insurance companies (payers) and other entities that gather information about therapeutic effectiveness of drugs in everyday clinical practice (such as prospective observational studies), electronic medical records,National Health Fund (NFZ) reports, registers of epidemiological data, or questionnaire-based studies of patients.
For obvious reasons, scientific quality and validity of information generated from RWE depend on a number of factors, including data quality (completeness and representativeness), or the level of standardisation and clarity of the parameters measured (e.g. the underlying disease and its consequences). What is noteworthy, patient registers have a very special place among the sources of RWE as they meet both of these criteria.
For obvious reasons, scientific quality and validity of information generated from RWE depend on a number of factors, including data quality (completeness and representativeness), or the level of standardisation and clarity of the parameters measured (e.g. the underlying disease and its consequences). What is noteworthy, patient registers have a very special place among the sources of RWE as they meet both of these criteria.
Implementation of well-designed RWE registers translates into benefits in many aspects of healthcare and paves the way for more general use of this data in:
- everyday medical practice – the data collected help evaluate real patient benefits of various treatment options,
- assessment of medical technologies in cases where clinical trials are difficult to conduct / impractical / unethical,
- research projects – practical treatment outcomes can supplement the existing body of evidence from clinical, epidemiological, and many other fields of science, such as health economics,
- agreements between payers and pharmaceutical companies under outcome-based risk-sharing arrangements to attain maximum control over reimbursement costs.
|
Registers can help evaluate the effectiveness and safety of therapy, identify patient needs, supplement HTA report, and support evidence-based reimbursement decisions, investigate the overall effectiveness of the healthcare system, or implement commercial and marketing strategies. |
Current state and future perspective of RWE in Poland
Identified barriers
In Poland, there is limited access to data that meet the criteria of RWE. The existing standardised registers, records, lists, inventories, or other structured sets of medical data are maintained mainly for settlement of accounts with the National Health Fund (NFZ). This entails a relatively narrow scope of information collected (in population and qualitative terms), data fragmentation among thousands of healthcare providers, and lack of surveillance over the scientific value of the registered data, all of which translates into poor practical value and limited usability of such data.
There are very few comprehensive medical registers in Poland, as they are typically limited to highly selected populations of patients or health conditions. These are mainly registers maintained by the Ministry of Health, e.g. the National Cancer Register (KRN), the National Cardiac Surgery Register (KROK), the Polish Register of Acute Coronary Syndrome (PL-ACS), and the relatively recent Medically Assisted Procreation Register and the Register of Non-malicious Large Salivary Gland Tumours.
NFZ also creates comprehensive medical databases, such as the Disease Treatment Register (RLC) and the Therapeutic Programs Monitoring System (SMPT) – these are dedicated modules of the NFZ IT systems where selected data for individual diseases is stored (i.e. solely the data relevant for and limited to NFZ-funded healthcare services, structured according to the ICD-10 code, treatment used, or clinical parameters belonging to a particular drug program, etc.).
Scientific associations also make attempts to collect data (for example, under the project "Long-term Safety and Effectiveness Assessment of Therapies used in Juvenile Idiopathic Arthritis" initiated by the Polish Rheumatic Disease Association).
The conditions for exploring the potential afforded by RWE have changed markedly with the introduction of a new drug reimbursement system. According to the new Drug Reimbursement Act [3] effective since 2012, "efficacy and effectiveness" are one of the criteria considered in deciding whether new medical technologies (and medicinal products) are eligible for reimbursement or not, and in consequence the new law makes room for therapeutic effectiveness data in the overall health technology assessments (HTA) system. The drug reimbursement law also mentions outcome-based pricing schemes as one of risk-sharing arrangements between the Ministry of Health and pharmaceutical companies, as part of the enrolment process of new medicinal products into reimbursement lists. However, public institutions have yet to take practical steps to implement these solutions.
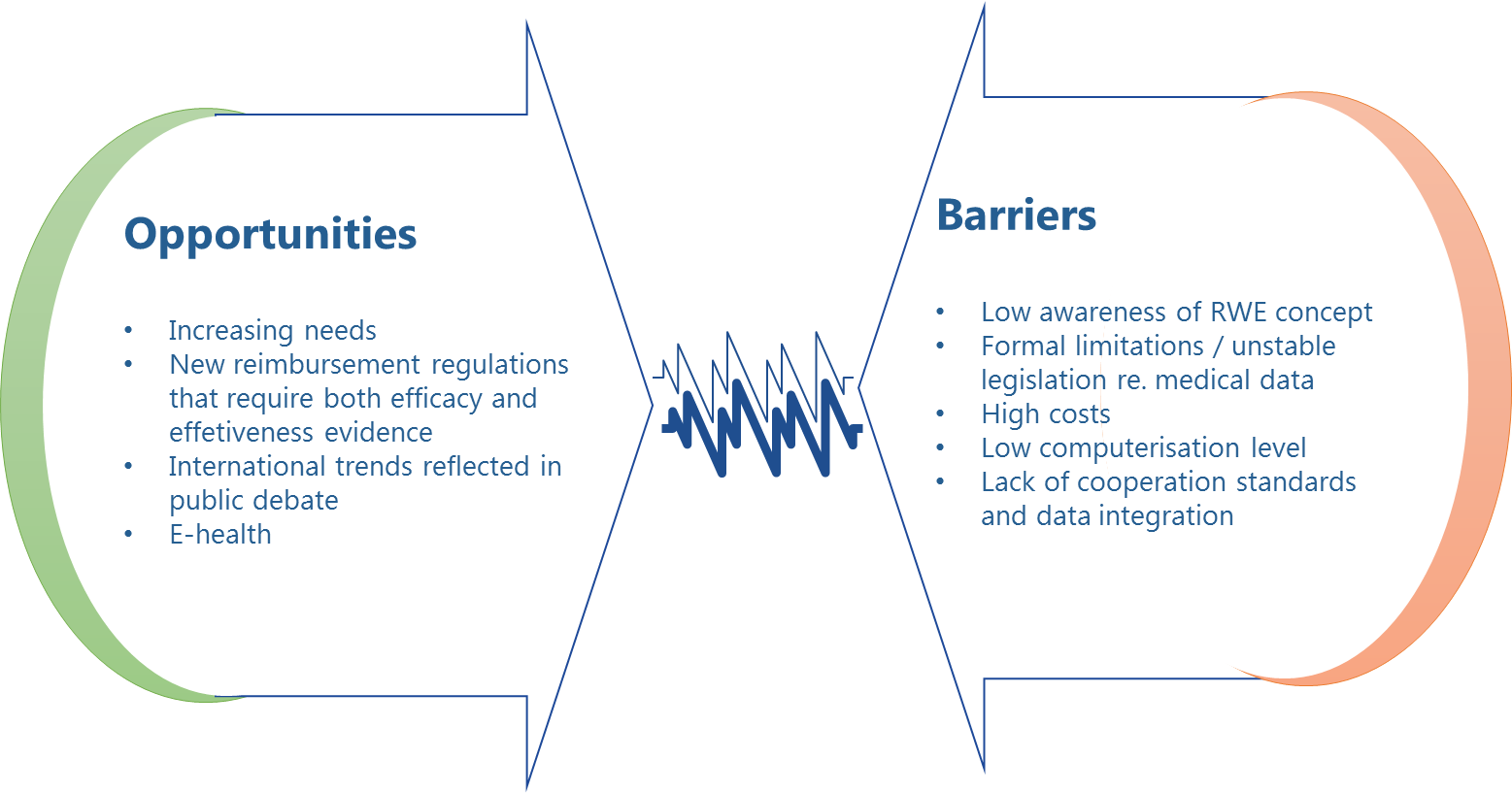
In general, RWE development in Poland is being slowed down by a variety of obstacles. One crucial barrier is low awareness about RWE, and specifically about the benefits of collecting and analysing real world data, and about the needs RWE could satisfy, especially among top-level decision-makers in the healthcare sector. Healthcare experts and the academic community, on the other hand, seem fully aware of the significance of RWE. In addition to research work based on data collected in Polish medical registers, there is also an increasing number of academic articles and public debate that bring the topic of RWE closer to the attention of decision-makers, advocating for more registers to be created in Poland, not only for the sake of actively taking part in the scientific development worldwide, but also in the hope for new system-wide solutions in Poland [4-6].
Another important issue involves formal limitations in medical data collection and processing arising from legal restrictions related to personal data protection and the resulting controversies over the ownership, processing, and dissemination of medical information. In accordance with legal provisions in force in Poland [7], processing of medical personal data is only permitted under explicitly defined circumstances and, as a rule, subject to the written consent of the data subject, except where otherwise stated:
- in separate regulations, for example those pertaining to the National Health Fund (NFZ) or the Social Security Office (ZUS),
- to protect the health status, provision of healthcare services, or patient treatment delivered by healthcare entities,
- for the purposes of scientific research (in a scientific article, personal data must be anonymous).
It seems most desirable for public medical registers to be set up on the basis of laws and regulations, in which case no consent would be mandatory from data subjects (which would also ensure sample representativeness and high quality of statistical data); it would also guarantee completeness and effectiveness of data collection by imposing a statutory obligation to report data in the circumstances set forth in the relevant act, although this solution may be considered less favourable given the slow and complex legislative process in Poland.
In an attempt to accelerate the development of RWE, at some point registers were allowed to be created on basis of MoH regulations [8]. However, there was considerable uncertainty as to the outcome of this legal solution, and in December 2014, the Constitutional Tribunal declared the new rules unconstitutional.
The relatively low levels of computerisation of healthcare settings in Poland and restricted exchange of information among central systems (NFZ, KRN, and ZUS, etc.) present further obstacles to more extensive RWE collection on a nation-wide scale. Despite the comprehensive legislation adopted in 2011 [8] designed to reorganise the existing system for healthcare data collection, processing, and use, which was intended to streamline the development of e-health in Poland and to create a stable nation-wide information system – the strategy of upgrading the IT infrastructure and improving medical information exchange is still in its implementation phase. For example, the deadline for an obligatory transition from traditional to electronic form of medical records has been postponed few times. The implementation level of systemic solutions in this area, marked by lack of uniform standards for data content and quality review, or data exchange regulations and tools, directly translates into the degree of utilisation of the RWE potential.
Finally, creating databases (registers) requires considerable financial and organisational effort, which creates another significant impediment to the effective development of RWE in Poland, further aggravated by low awareness about RWE benefits on the one hand, and limited resources of the public healthcare system on the other hand.
Potential solutions
One solution to reduce obstacles to the development of RWE in the Polish healthcare system would be to establish rules and solutions based on multi-institutional partnerships among stakeholders involved in collecting and analysing real world medical data, as is the case in many European countries. For example, many European registers of therapies used in cancer and rheumatic diseases are maintained by scientific associations that cooperate with academia, while at least part of the funding is provided by pharmaceutical companies under terms and conditions similar to research grants [5,6].
Similar cooperation standards between the stakeholders (academia, governmental institutions, including MoH, Office for Registration of Medicinal Products, NFZ, and ZUS, commercial payers and insurers, and pharmaceutical companies) are still missing in Poland. As a result, the medical data collected and processed in Poland as part of the existing system has not been properly integrated; it is incomplete and difficult to access by the healthcare stakeholders, such as medical centres or institutions in charge of monitoring the healthcare system in Poland and the quality of medical services.
Restricted access to information offers little opportunities to compare clinical, epidemiological or cost indicators and parameters of the healthcare system from the geographic, patient and population point of view. In general, the existing registers, such as the SMPT (i.e. registers dedicated to detailed monitoring of drug programs by NFZ) should provide ample opportunities to control the size and selection of populations belonging to particular drug programs, to regularly assess and trace the program outcomes, and to monitor and evaluate indicators describing the effectiveness of drug technologies. However, such opportunities should not be limited to the institution that administers and collects the data concerned, not only on grounds of substance and for social reasons, but also from the sake of transparency in public spending.
The following solutions can help improve the status of medical registers in the healthcare system in Poland:
- Transforming the existing databases (e.g. SMPT) so that they collect and evaluate health outcomes for reimbursed drugs, involving risk sharing schemes and outcomes based agreements;
- Enabling private entities to get paid access to anonymised medical databases or registers, drawing on the experience from Hungary, Czech Republic, Sweden, or the UK. Paid access to statistical data would further improve data quality and stability of such initiatives in the long term perspective;
- Promoting public-private partnerships (between e.g. broadly understood payer or decision-maker and pharmaceutical companies, academia, healthcare providers) in terms of factual and financial involvement in setting up and maintaining medical registers.
- Setting up a group of experts bringing together representatives of payers, decision-makers, academia, patient organisations, and the pharmaceutical sector to work on the methodological assumptions for analysing and publishing data from medical registers or drug programs based in Poland.
- Setting up a strategic and analytical structure at the Centre of Health Information Systems (CSIOZ) to continuously analyse and present broadly defined aggregated medical data and to cooperate with the established players in the healthcare sector in the scope of commercial exchange of information.
Development of and access to RWE in the current legislative and structural framework in Poland largely depends on whether all stakeholders are able to take steps to:
- identify the demand of all healthcare system stakeholders for real-world evidence;
- develop rules for collecting and processing of data, and create tools and infrastructure dedicated to this process;
- the establishment of dialogue to set up transparent and functional rules of cooperation among stakeholders to achieve optimum use of the existing resources.
In order to implement these measures, it is necessary to be aware of the benefits of RWE-based data analyses as a 'multifunctional' source of information for the healthcare system.
|
Restricted RWE use in Poland is a consequence of the current regulatory framework, low levels of financial and IT resources in the healthcare sector, and lack of cooperation patterns among stakeholders. This is why it is particularly important to identify the demand for and raise awareness about the potential afforded by RWE among all stakeholders, which will hopefully pave the way for a strategy of RWE development and improvement of access to data. |
Demand for RWE among medical practitioners
Medical practitioners are important stakeholders involved in determining the future of RWE in Poland. These are the main beneficiaries of RWE used in professional development and in advancing the quality of therapy. So far in Poland we have already seen a dialogue among experts, along with some organisational changes in the Polish healthcare system influencing the shape and access to RWE in Poland; however, the attitude to and the demand for RWE among the key stakeholders – medical practitioners – are not yet known. Hence arose the need for a study to diagnose the demand for specific types of RWE and the awareness of the potential offered by RWE among medical practitioners in Poland.
|
Medical practitioners are one of the main beneficiaries of RWE. Still, their awareness and needs related to RWE have not been explored. |
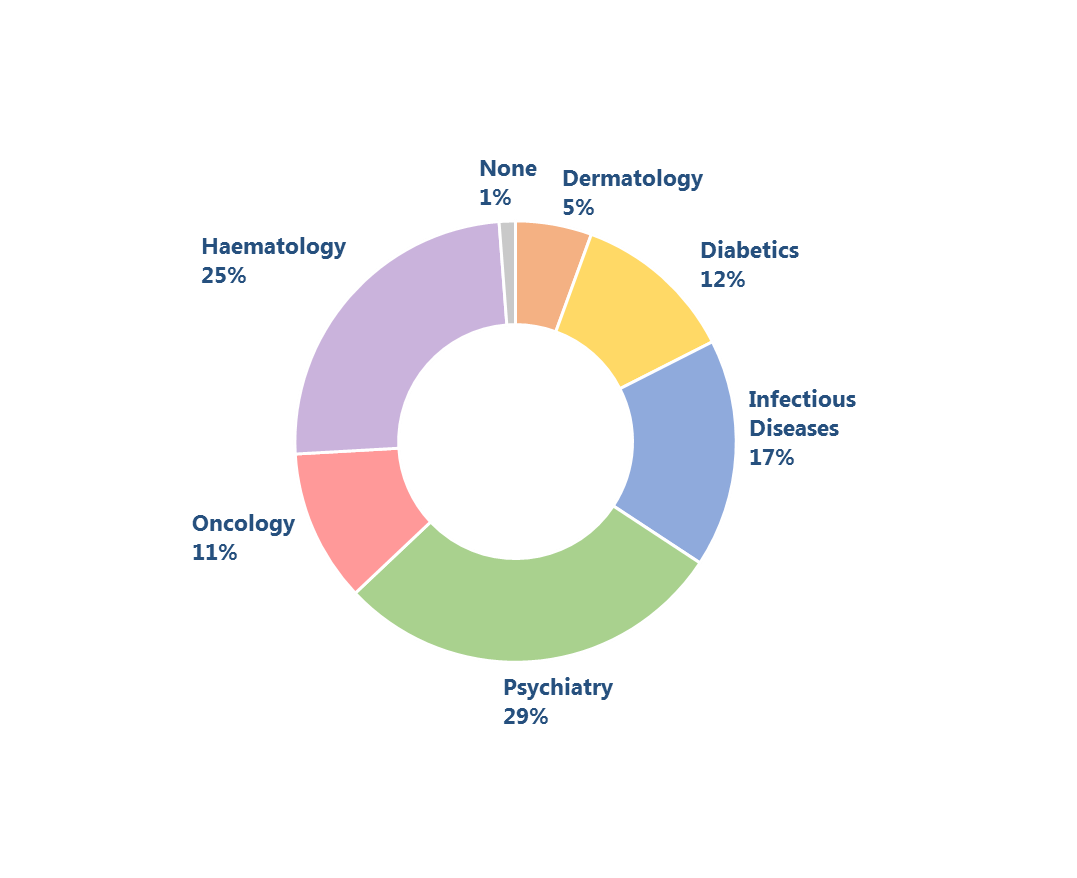
The study was conducted all over Poland among medical practitioners from across different medical specialised areas: diabetics, infectious diseases, psychiatry, oncology, haematology, and a few non-specialised physicians working in outpatient and/or inpatient settings. The survey questionnaire included qualitative and quantitative questions on a large scope of topics: demand for real world data concerning treatment outcomes, interest in RWE data, or the most reliable sources of RWE information in Poland. The questionnaires were collected in the period from September to November 2014. A total number of 251 responses were received. Data was analysed using descriptive statistics.
The study broadly indicates that medical practitioners are generally dissatisfied with the level of access to real patient outcomes. Up to 90% of respondents declared they lacked access to real world evidence, and only 10% of the medical practitioners surveyed expressed the opposite opinion.
Most respondents stressed the importance of information directly related, but not limited to the practical aspects of therapy. For example, one out of five diabetologists chose "epidemiological data", both at national and regional scale, as the most important type of real world evidence they often missed in their respective field of specialisation. Haematologists and oncologists expressed similar opinions about "lack of registers" that might be used to analyse therapeutic patterns and treatment options in individual healthcare centres. Absence of "system-wide registers of drugs used by patients" has been brought up by oncologists. Diabetologists, psychiatrists, and infectious disease (ID) specialists shared a broader view on the demand for RWE information, declaring that they needed "access to follow-up information from other physicians, for example general practitioners" (diabetologists), "follow-up data – how the patient performed outside the outpatient settings (in the society)" (psychiatrists), "data about the health status of patients 'cured' more than 3 years before" (ID specialists). Some of this information has been systemically collected by NFZ and other healthcare institutions; however, access to this data is more problematic. The replies to the questionnaire show a broad spectrum of needs and interests of medical practitioners in this area.
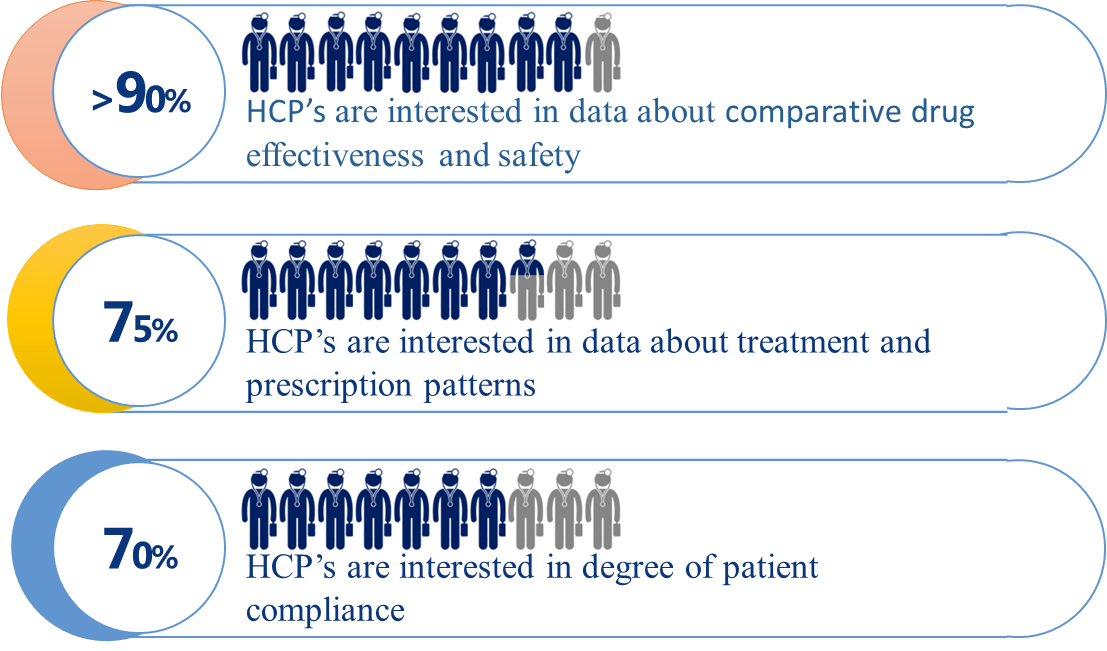
When asked about the most desirable RWE data, the vast majority of respondents – over 90% – declared they were particularly interested in information about safety and comparative effectiveness of therapies. This clearly indicates that – from the perspective of medical practitioners – system-based registers collecting real world data should be primarily a source of information about therapeutic safety and effectiveness, complementary to the results of controlled clinical trials and observational studies.
|
The vast majority of respondents were not satisfied with the level of access to real world treatment outcomes. In everyday medical practice, doctors were found to be very much in demand for comparative data about the safety and effectiveness of various therapies (new drug vs. new drug, instead of new drug vs. old standard). |
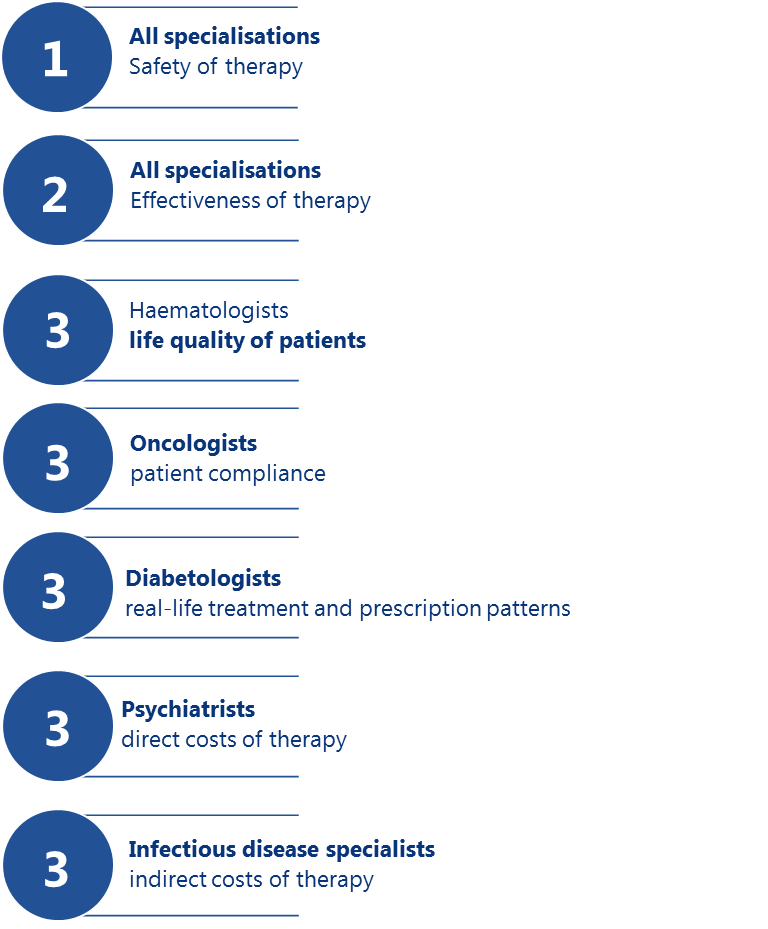
The respondents almost unanimously considered effectiveness and safety of therapy as two most important aspects of RWE. Still, respondents from various fields of specialisation had divergent preferences for other types of RWE. Diabetologists declared high interest in data about treatment and prescription patterns (87% responses), haematologists preferred quality life data (87%), oncologists opted for patient compliance data (79%), infectious disease specialists were interested in indirect costs of treatment (68%), and psychiatrists demanded more information about direct costs of treatment (69%).
The prevailing majority of respondents indicated that they considered scientific societies as the most reliable and useful source of RWE (80% of respondents). Another important source of reliable RWE were case reports published in medical journals (63% of responses), especially among medical practitioners working in inpatient settings (67% vs. 48% of responses among medical practitioners in outpatient settings). Practitioners in outpatient settings, as compared to inpatient care practitioners, were more likely to favour data from NFZ and ZUS, considering it highly reliable and useful. The lowest number of respondents listed as reliable data from market research and information provided by patient associations (26% and 20% respectively).
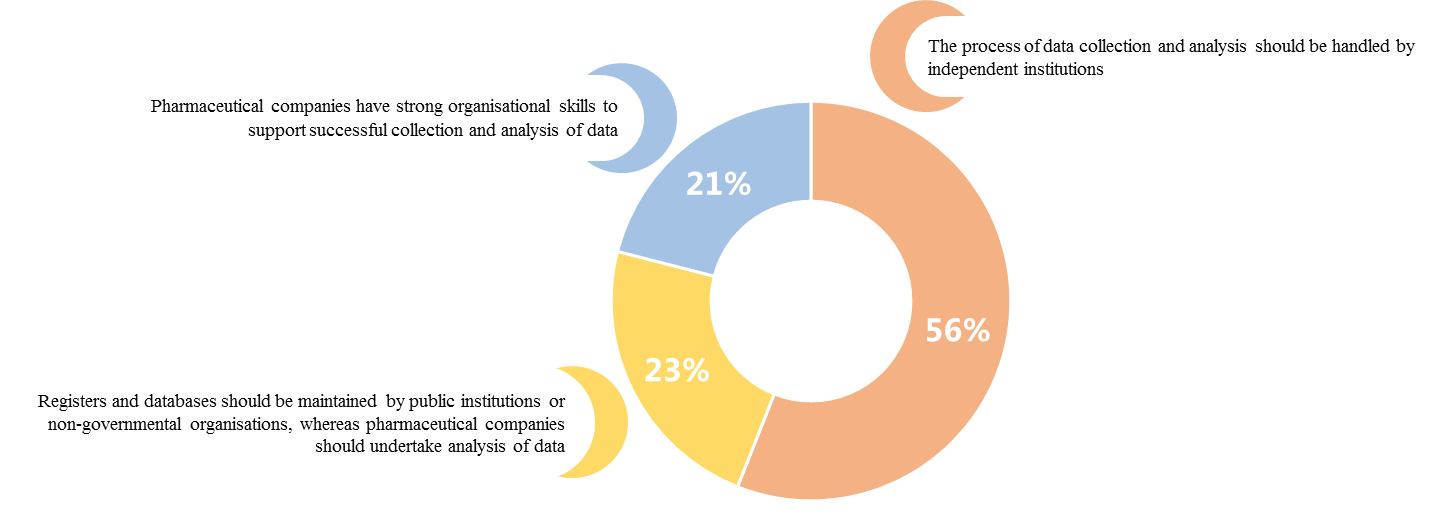
The survey also showed high support to the idea of creating RWE registers and RWE analyses carried out by independent institutions, set up either under the public law regimen or by non-governmental organisations.
The role of the pharmaceutical industry was also highly underlined.Respondents expected pharmaceutical companies to educate medical practitioners about RWE (80% of responses), share information about scientific publications based on RWE (51%), and partner with academia to raise the awareness of the scientific community about RWE (38%).
|
The medical practitioners surveyed declared that RWE registers should be maintained and the RWE data analysed by independent institutions. In the opinion of medical practitioners, pharmaceutical companies should spread knowledge about RWE and keep medical practitioners informed about new scientific publications based on RWE. |
Conclusions
The potential of real world evidence appear to be increasingly recognised in Poland, although Poland lags behind other countries when it comes to implementing solutions for collecting, processing, and dissemination of information about the actual effectiveness, safety, and costs of therapy or epidemiological data. While there are areas where some RWE-relevant solutions have been operating, systemic solutions that promote RWE are still missing. Restricted RWE use in Poland has many causes and is a consequence of the current regulatory framework, low level of financial and IT resources in the healthcare system, and lack of cooperation patterns among stakeholders.
This is why it is particularly important to identify the demand for and raise awareness about the benefits by RWE among all stakeholders, which will hopefully contribute to the establishment of a strategy for RWE development and improvement of access to data.
The awareness of and demand for RWE among medical practitioners remained largely unrecognised, although they are among the main stakeholders in this area. The survey highlighted that the majority of respondents were dissatisfied with the level of access to real world patient outcomes. Among those who declared they were missing RWE-quality information, the majority believed that data about the relative treatment effectiveness are difficult to access in Poland. Respondents also pointed out the scarcity of data about real-world costs of therapy, along with data on the patient quality of life and real-world treatment and prescription patterns. The missing data appears to be indispensable for taking evidence-based decisions about the most effective treatment strategies in everyday clinical practice or for monitoring patient outcomes. This leads to the conclusion that better access to RWE could have a positive impact on the decision-making process in all issues relating to patient therapy. The survey also proved that the need for information varies across different medical fields of specialisation, reflecting the specific nature of demand for information in various medical fields.
Medical practitioners in Poland were shown to have a clear opinion about the demand for and the range of data generated outside clinical trials. Systematic education about RWE among healthcare professionals and other stakeholders, dissemination of knowledge about the benefits of RWE, and better access to RWE are of key importance in setting up the framework for the development of RWE in Poland, and the resulting improvement in patient outcomes.
Effective healthcare management requires access to up-to-date and reliable data (ranging from epidemiological data to information about the actual effectiveness of medical technologies in everyday medical practice, etc.), which is still few and far between in Poland. Keeping RWE registers and most notably analysing and disseminating RWE are preconditions for improvement of the quality and effectiveness in healthcare. Real world evidence about patients in Poland and the Polish healthcare sector would not only encourage scientific research, but also provide a solid ground for rationalisation of healthcare expenses.
[1] The term Real World Evidence within the meaning of real-life data generated by and in connection with the healthcare system is used interchangeably with the term Real World Data (RWD) – this is the convention used in this document. In some articles and discussions about data collected in actual clinical practice, the term RWE has a narrower meaning to denote a subset of structured and validated RWD used in the decision-making process about drug reimbursement, development, or marketing, etc.
- Garrison LP. et al., Using Real-World Data for Coverage and Payment Decisions: The ISPOR Real-World Data Task Force Report. Value Health 2007; 10(5): 326-338
- Eichler HG. et al. Relative efficacy of drugs: an emerging issue between regulatory agencies and third-party payers. Nature Reviews Drug Discovery, 2010; 9(4): 277-291
- Act of 12 May 2011 on reimbursement of medicinal products, foodstuffs intended for particular nutritional uses and medical devices (Polish Journal of Laws 2011, No 122, item 696, as amended)
- Polish Diabetes Association. A call for support of the national program for prevention of diabetes and related complications. 19 May 2014; Available from: http://www.cukrzyca.info.pl/aktualnosci/apel_o_popracie_narodowego_programu_przeciwdzialania_cukrzycy_i_jej_powiklaniom; [Accessed: 30.01.2015]
- Raciborski F. et al. Rejestry medyczne w reumatologii: czy w Polsce potrzebny jest rejestr reumatologiczny? Reumatologia 2012; 5(50): 416-424
- Zyśk R., Krzakowski M. Możliwości poprawy systemowego dostępu chorych do innowacyjnych metod przeciwnowotworowego leczenia w Polsce. Nowotwory; Journal of Oncology 2014 Special Edition; 20-25
- Act of 29 August 1997 on the protection of personal data, as of 26 June 2014 (Polish Journal of Laws 2014, item 1182)
- Act of 28 April 2011 on the healthcare information system (Polish Journal of Laws No 113, item 657, as amended)
- Polygenis D., ed. ISPOR Taxonomy of Patient Registries: Classification, Characteristics and Terms. Lawrenceville, NJ; 2013
- Gliklich RE., Dreyer NA., eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Prepared by Outcome DEcIDE Center. AHRQ Publ. No. 07-EHC001-1. Rockville, MD: Agency for Healthcare Research and Quality, April 2007 (2nd ed,). September, 2010
- Noe L. et al. Utilizing Patient Registries to Support Health Economics Research: Integrating Observational Data with Economic Analyses, Models, and Other Applications. The Official News & Technical Journal of the International Society for Pharmacoeconomics and Outcomes,Research. ISPORconnections,2005; 11(5):15