Is the cost-effectiveness threshold cost-effective in cancer therapy?
-
Copyright
© 2015 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Łukasz Kaczyński |
Aestimo s.c., Kraków, Polska |
|
Beata Serafin |
Aestimo s.c., Kraków, Polska |
|
Patrycja Prząda-Machno |
Pfizer Polska, Warszawa, Polska |
|
Marcin Kaczor |
Aestimo s.c., Kraków, Polska; Uniwersytet Jagielloński Collegium Medicum, Kraków, Polska |
Introduction: To compare availability of innovative cancer drugs in countries with established cost-effectiveness threshold (Great Britain, Czech Republic, Sweden and Poland) and where this criterion is not used (Germany, France, Italy), and to assess the relationship between the reimbursement system and the expenditures and health outcomes.
Methods: The analysis of reimbursement decisions concerning innovative cancer drugs approved by the European Medicines Agency in the years 2012-2013 and the available data concerning incidence, 5-year relative survival rate and the treatment costs of breast, lung, colon and prostate cancer.
Results: The assessment included 19 innovative drugs. Most of them were covered by reimbursement in countries without a cost-effectiveness threshold — 80.7%, compared to the countries with a cost-effectiveness threshold — 59.6% (48.7% including Poland). Three products were financed in all reimbursement systems, with the exception of Poland: Perjeta, Xalkori and Xtandi. In countries without the cost-effectiveness threshold the health outcomes expressed as 5-year relative survival were better compared to EU in general or to most of countries which use the cost-effectiveness threshold, whereas expenditures per capita in most cancers were diversified — large (15-27 €, Germany) or similar to the countries with the cost-effectiveness threshold (6-13 €, France and Italy). In Poland for most evaluated cancers the lowest 5-year relative survival was noted, with the lowest expenditure per capita (5-9 €) simultaneously.
Conclusions: The percentage of reimbursed innovative cancer drugs is higher in countries without the cost-effectiveness threshold, where better health outcomes are observed. Limited access to innovative therapies resulting from restrictive financial criteria may have a significant impact on health outcomes in cancer treatment.
Introduction
The value of the incremental cost-effectiveness ratio (ICER) is one of the criteria considered when undertaking a decision on drugs reimbursement. For example, in Poland the cost-effectiveness threshold for gaining one quality-adjusted life year (QALY) is equal to three times the Gross Domestic Product (GDP) per capita. On the other hand, in many countries of Western Europe there are no strict cost-effectiveness threshold values defined, and the reimbursement decisions are based mainly on the quantification of a health benefit and on the size of the target population.
In case of innovative medicinal products used in cancer therapy, meeting the requirement of cost-effectiveness not exceeding the set cost-effectiveness threshold is particularly difficult to achieve, since in limited target population drug prices that provide manufacturer a return on the high expenditure on research and development are usually high [1]. Therefore in countries which use the cost-effectiveness threshold, alternative paths of reimbursement are being used to increase access to modern cancer therapies [2].
According to published data the reimbursement criteria have an impact on the availability of new cancer drugs; however, this source refers mainly to therapies registered by European Medicines Agency (EMA) before 2012 and there is no information for Poland [3].
The aim of this paper is to compare the availability of innovative cancer drugs in countries which use the cost-effectiveness threshold in the reimbursement process (including Poland) with the countries where this criterion is not used, and to assess the relationship between the reimbursement system and the expenditures and generated health outcomes.
Methods
In order to assess the degree of reimbursement of modern drugs for cancer patients in the two analysed models of reimbursement decisions making, 7 countries were selected. The group of countries with the cost-effectiveness threshold includes the Czech Republic, the United Kingdom, Sweden and Poland, and the group of countries where the threshold for gaining an additional quality-adjusted life year is not a criterion for reimbursement includes — France, Germany and Italy. Regarding the countries with the cost-effectiveness threshold its value was calculated from the national currency into PLN using the average National Bank of Poland currency exchange rate for the 1st half of 2015.
The analysis included innovative cancer drugs approved by EMA in the years 2012-2013. The drugs were selected by searching the EMA websites for positive approvals for new anticancer drugs, excluding generic drugs, drugs with approved label extension or drugs with approved label change. Subsequently, it was checked whether the identified anticancer therapies were also assessed by the Polish Agency for Health Technology Assessment and Tariff System (AOTMiT, Polish Agencja Oceny Technologii Medycznych i Taryfikacji). The reimbursement status of the identified drugs was verified on the lists of reimbursed products in individual countries (cut-off date: 15th October 2015).
To illustrate the association between the used reimbursement model and the epidemiological indicators as well as expenditures, 4 most frequent cancers in Poland in 2012 were selected: lung cancer, breast cancer, colon cancer and prostate cancer [4]. The values of 5-year relative survival were obtained from EUROCARE-5 retrospective study, where relative survival of patients was calculated using the data from patients diagnosed between years 2000-2007, and followed until 2008, in 107 national cancer registries from 29 European countries. Relative survival is the ratio of cancer patients, who remain alive after a specified period, compared to the survival of general population with similar demographic characteristics (age, gender, residence). This rate took into account the increase of mortality resulting from the cancer diagnosis, but was not adjusted for the cancer’s stage at diagnosis [5]. Incidence rates were obtained from the Internet website of the GLOBOCAN project, which presents the information on incidence, mortality and morbidity in 184 countries (state as of 2012), based on the data provided by International Agency for Research on Cancer, data published in the Internet and local sources [6]. Costs of cancer treatment were taken from the Luengo-Fernandez 2013 study, which presented the costs of cancers in 27 European Union countries (mostly from 2009), with purchasing power parity method (taking into account the price differences in individual countries). The study authors have included 5 categories of costs: primary care, emergency care, outpatient care, inpatient care and drugs. The indirect costs of unpaid care provided to the patient by family members and/or friends, income loss after premature death or unemployment caused by a chronic disease were additionally taken into account [7].
Results
Cancer drugs reimbursement models in selected European countries
In some European countries there are no formally required cost-effectiveness thresholds or they are not an absolute criterion for reimbursement decisions, which are made mainly based on additional health benefit generated by the assessed technology. Such solutions are used e.g. in France, Germany and Italy.
In France, the health technology assessment is conducted by the National Authority for Health (HAS, French Haute Autorité de Santé) created in 2004. The Transparency Committee (CA, French Commission de la Transparence) acting within HAS issues an opinion on the Medical Benefit (SMR, French service médical rendu) and the Improvement of Medical Benefit (ASMR, French amélioration du service médical rendu) related to the new medical technology, which applies for reimbursement. The SMR and ASMR assessment is performed based on available literature data: randomized studies, meta-analyses, recommendations, surveillance studies and pharmacovigilance. The detailed conditions for reimbursement, including the drug price, are established during negotiations between the Economic Committee for Health Products (CEPS, French Comité Economique des Produits de Santé) and the manufacturer. The ASMR is the key aspect taken into account during financial negotiations. The price of the reimbursed medicinal product should not be lower than the lowest of its prices in Germany, Spain, Italy or the United Kingdom. Final decision on including the drug on the list of reimbursed medicinal products is made by the Minster of Health. France has no exceptions from the standard reimbursement procedure [8,9].
Whereas in Germany the drugs are usually reimbursed immediately after being authorised by the European Medicines Agency. In accordance with the amendment of the Act on the Reform of the Market for Medicinal Products (AMNOG, German Arzneimittelmarkt-Neuordnungsgesetz), adopted in 2011, during the first 12 months after a new product’s launch the marketing authorisation holder may set its price arbitrarily. Over this time the drug is included in the reimbursement process — an assessment of the advantages of its use is compared to the existing standard procedure, based on the health technology assessment (HTA) report provided by the drug company, which includes an obligatory systematic review. The scientific Institute for Quality and Efficiency in Health Care (IQWiG, German Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen) in cooperation with the Federal Joint Committee (G-BA, German Gemeinsamer Bundesausschuss) is responsible for clinical and cost effectiveness analyses of the new drug. The reimbursement conditions are established by the Central Federal Association of Health Insurance Funds (GKV-SV, German GKV-Spitzenverband) depending on the additional health benefits obtained. The price of the drug is established based on 3 parameters — additional health benefits related to the drug, the annual costs of comparator therapy and the level of drug price in Europe depending on the turnover volume and retail prices. For orphan drugs the German system differs from the standard reimbursement procedure — it is considered that the additional benefit emerging from the use of the drug was proven at the registration process stage. This principle does not apply to orphan medicinal products, which achieve an annual revenue of more than €50 million [9-14].
In Italy the reimbursement assessment and decision for new drugs is a task of the Italian Medicines Agency (AIFA, Italian Agenzia Italiana del Farmaco). To initiate drug assessment process the pharmaceutical company must submit a reimbursement application, containing the Summary of Product Characteristics, European Public Assessment Report (EPAR) and an EMA approval certification document. In accordance with the Italian criteria the drug is cost-effective when it is effective in a therapeutic area where there is no other treatment option, or where there are therapies available, but their effectiveness is insufficient, and in case of an advantageous cost/effect ratio when compared to other therapies for the same indication. The detailed reimbursement conditions are established during the negotiations conducted between the pharmaceutical company’s representatives and the Prices and Reimbursement Committee (CPR, Italian Comitato Prezzi e Rimborso) and the Technical Scientific Committee (CTS, Italian Commissione Tecnico Scientifica), acting as a part of AIFA. When establishing the drug price the average product price in Europe and the expected sales volume are considered. An exception from the standard reimbursement procedure is the possibility of filing an application for reimbursement for an orphan drug before the pharmaceutical company receives an official approval, which is intended to hasten the reimbursement procedure [9,15].
In some European countries the monetary value of the health benefit of the assessed medical technology (ICER) is one of the key criteria for establishing whether a reimbursement decision will be issued.
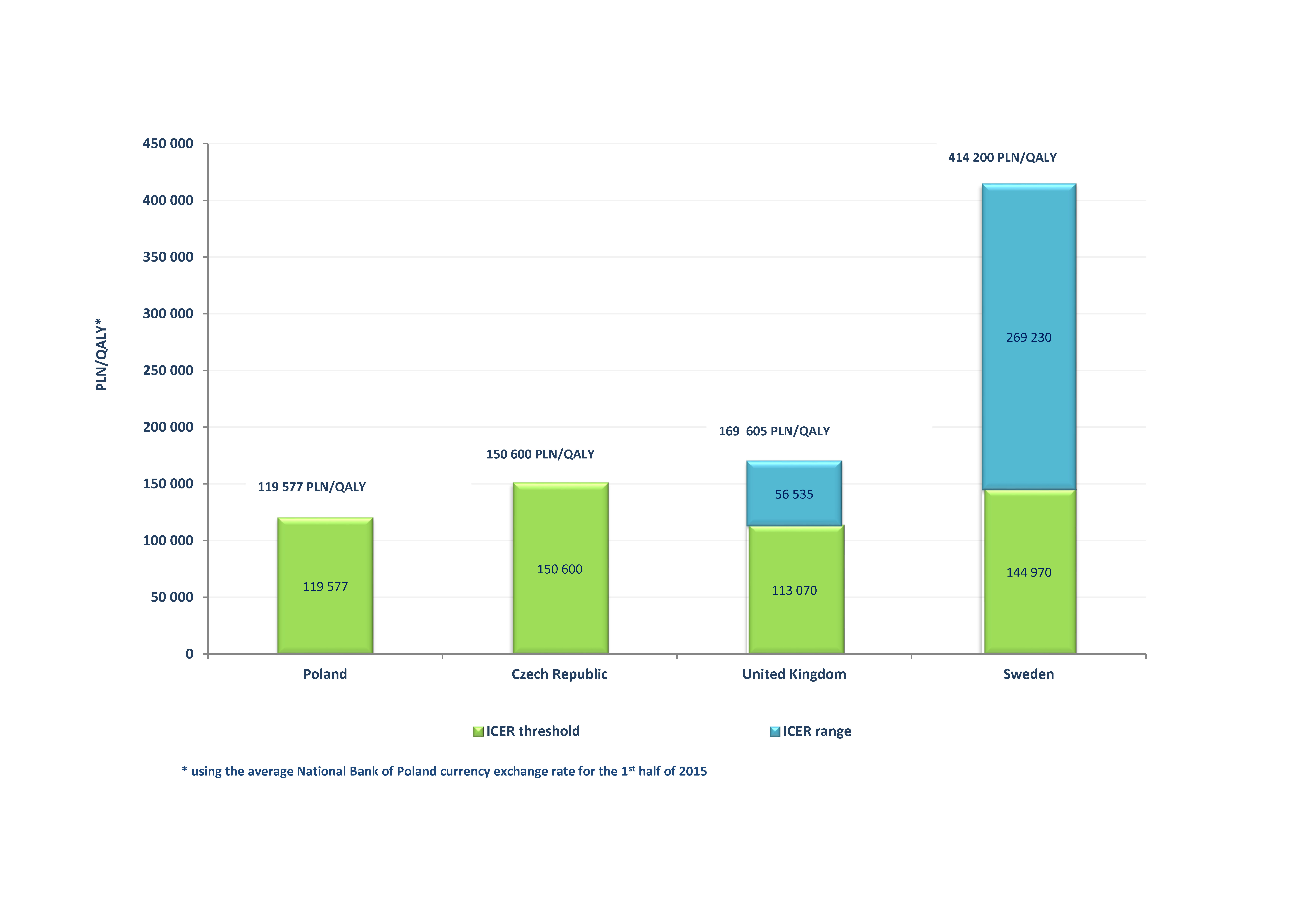
An example of such a reimbursement system is Poland, where the assessment of the justification of financing of drugs from the public funds is conducted by the Polish Agency for Health Technology Assessment and Tariff System (AOTMiT) — its decision, in the form of an opinion issued by the Transparency Council and of a recommendation of the Agency President is presented to the Ministry of Health. A leading factor that impacts a reimbursement decision is the cost-effectiveness of the given technology, defined as the cost of an additional quality-adjusted life year gained which should not exceed three times the GDP per inhabitant (119,577 PLN/QALY, condition per October 2015). The Economic Committee acting within the Ministry of Health conducts negotiations that focus on defining the reimbursement conditions, including setting of the official sales price. Since the 1st of January 2015, that is, since the possibility of applying for reimbursement of innovative cancer therapies as a part of non-standard chemotherapy was abolished, there are no separate reimbursement criteria for this group of drugs in Poland [16,17].
In the Czech Republic the responsibility for the reimbursement decisions and for setting the drug prices remains with the State Institute for Drug Control (SUKL, Czech Státní ústav pro kontrolu léčiv). In the process of issuing a reimbursement decision SUKL takes into account several indicators, including: HTA report (with a safety and efficacy assessment, as well as the cost-effectiveness analysis and the budgetary impact), severity of disease, public interest, or the possibility of replacement by another therapy. The maximum (ex-factory) drug price is established at the level of average of 3 lowest ex-factory prices of the product in the European Union (EU) (excluding Austria, Bulgaria, Cyprus, the Czech Republic, Estonia, Germany, Luxembourg, Malta and Romania). An exception is made for highly innovative technologies, in case of which the price information from the two reference EU countries are sufficient as a reference point. If the product is not available in at least 3 countries, the ex-factory price is established at the level of maximum drug price most comparable with the assessed technology available within the Czech Republic or the EU [9,18,19]. In the Czech Republic the threshold of the cost of quality-adjusted life year gained was set at the level of three times the GDP per capita [19]. The cost-effectiveness threshold is estimated at approximately 1,000,000 Kč/QALY (150,600 PLN/QALY) [20].
In the United Kingdom the main criterion taken into account during the process of reimbursement decision making is also the drug’s cost-effectiveness [21]. The organization responsible for issuing the recommendations on the use of pharmaceutical products is the National Institute for Health and Care Excellence (NICE). The assessment of a new drug by NICE is based on data from many sources, including independent expert groups, pharmaceutical companies, clinical specialists or patients. Moreover, the applicant is required to deliver all the clinical results (published, unpublished, abstracts, confidential data, registry data) concerning the assessed medical technology [14]. NICE obligates the National Health System (NHS) to cover the costs of recommended therapies, however a negative assessment by NICE does not preclude the financing of a therapy from public funds. A cost-effective therapy is characterised by an ICER on the level not exceeding 20,000 £/QALY (113,070 PLN/QALY). Additionally, in case of innovative technologies or treatment which extends life in terminal conditions, the uncertainties concerning ICER estimation, or when the reimbursement of the drug will result in benefits from the perspective of social costs, it is possible to consider the therapy to be cost-effective with ICER at the level of 20,000-30,000 £/QALY (113,070-169,605 PLN/QALY) [22]. Moreover, in case of end of life technologies NICE has issued additional recommendations which enable the correction and increase of the cost-effectiveness threshold above 30,000 £/QALY (169,605 PLN/QALY). The term “end of life” was used to define medical technologies which extend life by at least 3 months for a population of patients under 7,000, with an expected short-term survival of no more than 24 months. In case when conditions for an end of life therapy are met, NICE considers: (1) the impact of giving greater weight to QALYs achieved in the later stages of terminal diseases, using the assumption that the extended survival period is experienced at the full quality of life anticipated for a healthy individual of the same age, (2) and the magnitude of the additional weight that would need to be assigned to the QALY benefits in this patient group for the cost-effectiveness of the technology to fall within the current threshold range [2,14,23]. Moreover, an alternative source for the financing of cancer drugs that were assessed by NICE as not cost-effective or were not yet assessed is the Cancer Drug Fund (CDF) budget [24]. The justification for financing of drugs as a part of CDF is assessed by an expert panel with point criteria. The considered criteria, depending on the available data, include clinical efficacy, survival, patient’s quality of life, safety profile, existence of an alternative medical technology, median drug cost per patient and the strength of scientific evidence. Only those medicinal products are financed which obtained a number of points exceeding a specific threshold value, established depending on the budgetary financial resources in a specified period [25].
In Sweden since 2002 the decisions concerning drug reimbursement have been made by the Dental and Pharmaceutical Benefits Agency (TLV, Swedish Tandvårds Och Läkemedelsförmånsverket). A medicinal product which aims for reimbursement must meet three basic principles: of human value (when making decision on reimbursement people may not be discriminated against because of gender, race, age, etc.); of need and solidarity (a priority in the reimbursement is given to drugs used to treat more severe diseases) and of cost-effectiveness (the expenditures for the reimbursement of a drug are reasonable from the medical, humanitarian and socio-economical perspective). An important aspect of the Swedish drug reimbursement system is the lack of financial negotiations between the pharmaceutical company and TLV — the drug price proposed within the reimbursement application (which contains an HTA report) is an integral part of the cost-effectiveness analysis. The cost-effectiveness of assessed therapy is estimated from a social perspective. Sweden has no formally established medical technology cost-effectiveness threshold value. The drug cost-effectiveness is assessed depending on the therapeutic area. Analysis of reimbursement applications from years 2002-2007 indicates that threshold value for cost-effectiveness was €35,000 (PLN 144,970) on average, however positive reimbursement decisions were also made in case of technologies with an ICER value of approximately 100,000 €/QALY (414,200 PLN/QALY) in case of more severe conditions [9,26,27].
Figure 1 presents detailed information concerning the cost-effectiveness threshold values in Poland, the Czech Republic, the United Kingdom and Sweden.
The common element of the reimbursement systems in Poland, the Czech Republic, the United Kingdom and Sweden is the obligation to present a cost-effectiveness analysis of the therapy. It is based on comparative analysis of the ratio of financial expenditure to health outcomes resulting from the use of the compared medical technologies. The results of cost-effectiveness analysis provide a scientific basis for making rational decisions on the use and financing of health care benefits [28]. In these countries the key criterion for reimbursement decisions is the value of cost-effectiveness threshold for gaining an additional quality adjusted life year (QALY). The World Health Organization recommends setting a cost-effectiveness threshold at a level of 1-3 GDP per capita [29]. However, the use of cost-effectiveness threshold has some limitations.
First, it should be noted that the value of the ICER estimated for cancer technologies is usually much higher than for other therapies. A good example is the value of the incremental cost-effectiveness ratio for cancer drugs in the United States, which amounts on the average to 138,582 $/QALY (median value: 55,500 $/QALY), whereas for drugs from other therapeutic areas it is equal to 49,913 $/QALY (median value: 31,000 $/QALY) [1]. Making reimbursement decisions solely based on cost-effectiveness of medical technologies from the perspective of the public payer excludes the impact of the disease’s costs, both those incurred on the patients and their families and on the entire society. The methodological restrictions resulting from the method of calculation of the ICER should also be noted. The QALY is the main parameter used in cost-effectiveness analyses. It is a standard measure which takes into account both the qualitative and quantitative elements of the result. This value is the resultant of life expectancy and life quality subjectively assessed by patients [30]. However, the use of QALY value in cancers is connected with many uncertainties. The EQ-5D questionnaire as a tool for the measurement of quality of life in adult cancer patients has relatively low sensitivity to changes in terminal conditions. There are many other questionnaires, e.g. SF-6D, HUI or questionnaires dedicated to specific diseases, which could be used jointly or instead of a generic questionnaire, thus increasing the chances to capture even small changes in the characteristic symptoms of the given disease [31]. On the other hand, various methods of measurement result in different outcomes being obtained. The results of a study of early arthritis patient’s preferences measured using two questionnaires, EQ-5D and SF-6D are a good example. The results obtained using the EQ-5D questionnaire are characterised by a higher average change with a greater variance compared to the values obtained using SF-6D, regardless of the direction of changes. After 12 months SF-6D was more sensitive to changes related to the improvement of the patient’s health compared to EQ-5D (0.83 vs 0.57). Whereas EQ-5D more clearly indicated the exacerbation of the patient’s condition (-0.20 vs -0.11) [32]. Validating the questionnaires assessing the quality of life by people from general population is also problematic. It is possible that these people will not be able to entirely understand the situation of cancer patients. On the other hand, cancer patients have tendencies to assign higher values to a specific health condition than people from the general population [31]. In addition to health condition classification systems there are also various methods of utility measurements, including a rating scale, standard gamble, patient trade off, pair comparison or time trade off (TTO) [33]. The assumptions of the TTO technique preferred by NICE for utility measurement in population of terminal patients seem not entirely met. The TTO method consists of establishing the time period which the patient in a specified state of health would agree to trade off life in exchange for the return to a higher state of health. One of key assumptions of this method is the “constant proportional trade-off”, which states that individuals are willing to trade a constant part of their estimated life expectancy to obtain a proportional improvement of the health-related quality of life (HRQOL), regardless of the number of life-years remaining. However, as indicated by empirical studies, patients with an estimated life expectancy below 1 year would not trade any part of their expected lifespan to increase its quality [31].
Assessment of the reimbursement status of innovative cancer drugs
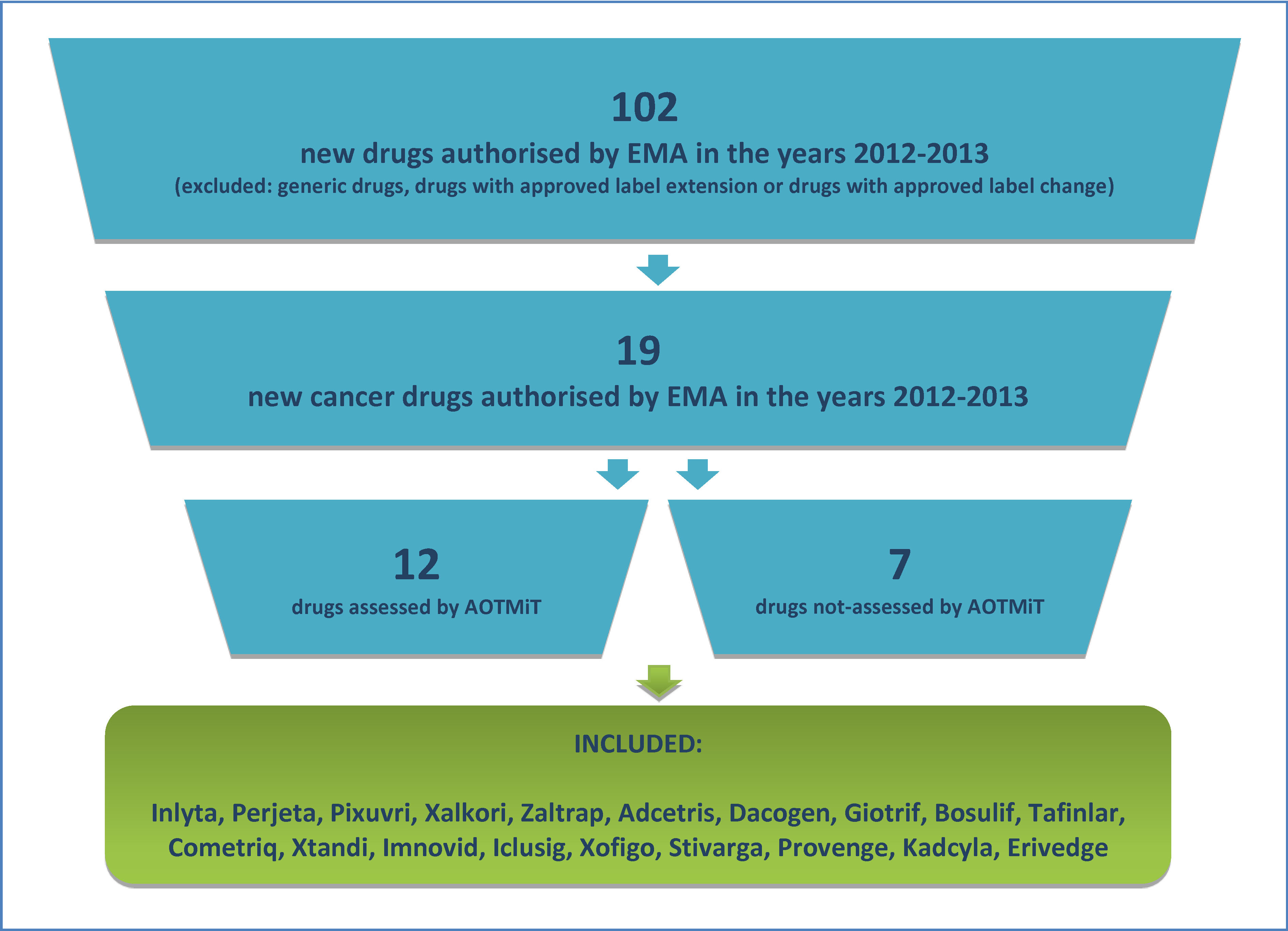
As a result of search, conducted through the European Medicines Agency website, it was established that in the years 2012-2013 this institution has issued positive decisions on the European Union approval for 102 new drugs, of which 19 (18.6%) were cancer therapies. The diagram of the process of drug selection for analysis was presented on Figure 2.
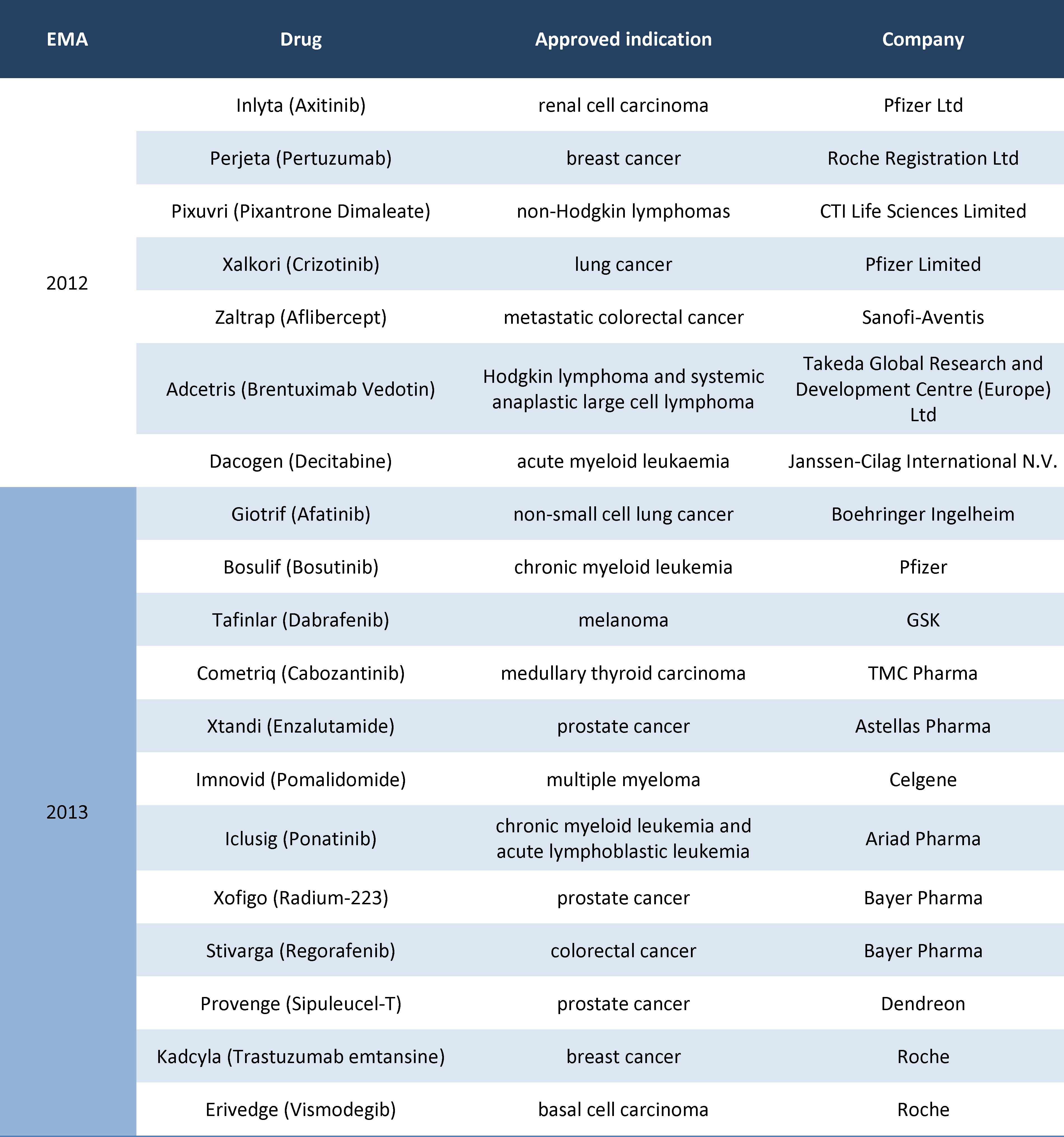
The Polish Agency for Health Technology Assessment and Tariff System (AOTMiT) has assessed 12 products (63.2%) of the cancer drugs approved for Europe in the years 2012-2013. Ultimately, all new cancer drugs authorised by EMA within the selected time period (Table 1) were included in this analysis.
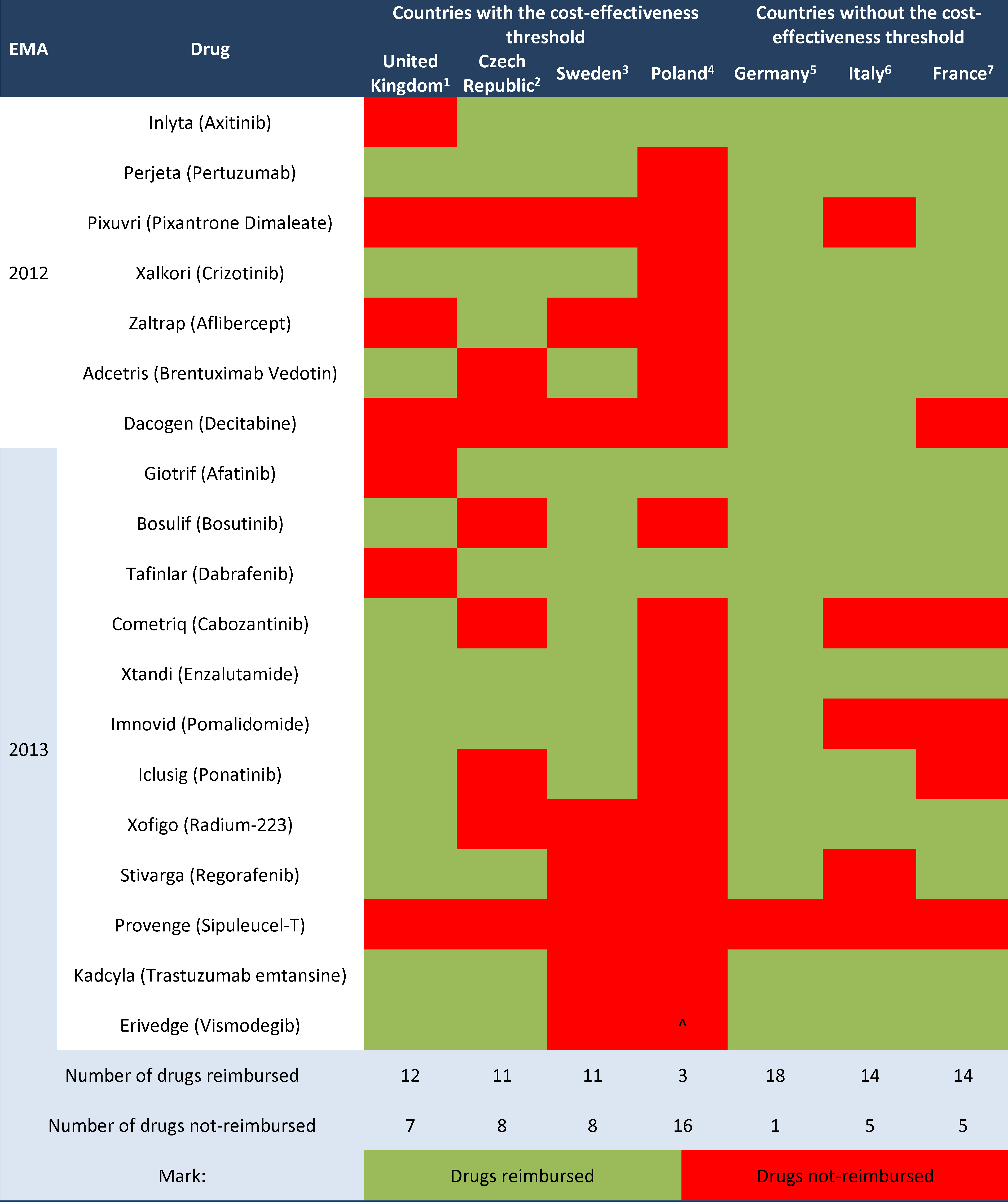
The financing of identified drugs from public means was analysed afterwards. Data from the selected European countries are provided in Table 2, marking the drugs available to patients within reimbursement programmes with green and non-reimbursed products with red.
Legend to Table 2.
1 http://www.england.nhs.uk/ourwork/pe/cdf/cdf-drug-sum/#sept;
2 http://www.sukl.eu/sukl/list-of-reimbursed-medicinal-products-valid-as-of-1-10-2015;
3 http://www.tlv.se/beslut/sok/lakemedel/;
4 http://www.bip.mz.gov.pl/;
5 https://www.gkv-spitzenverband.de;
6 http://www.agenziafarmaco.gov.it/it/content/liste-di-trasparenza-e-rimborsabilit%C3%A0;
7 http://base-donnees-publique.medicaments.gouv.fr/index.php;
^ drug not-reimbursed; on the AOTMiT website only the Verification Analysis is available.
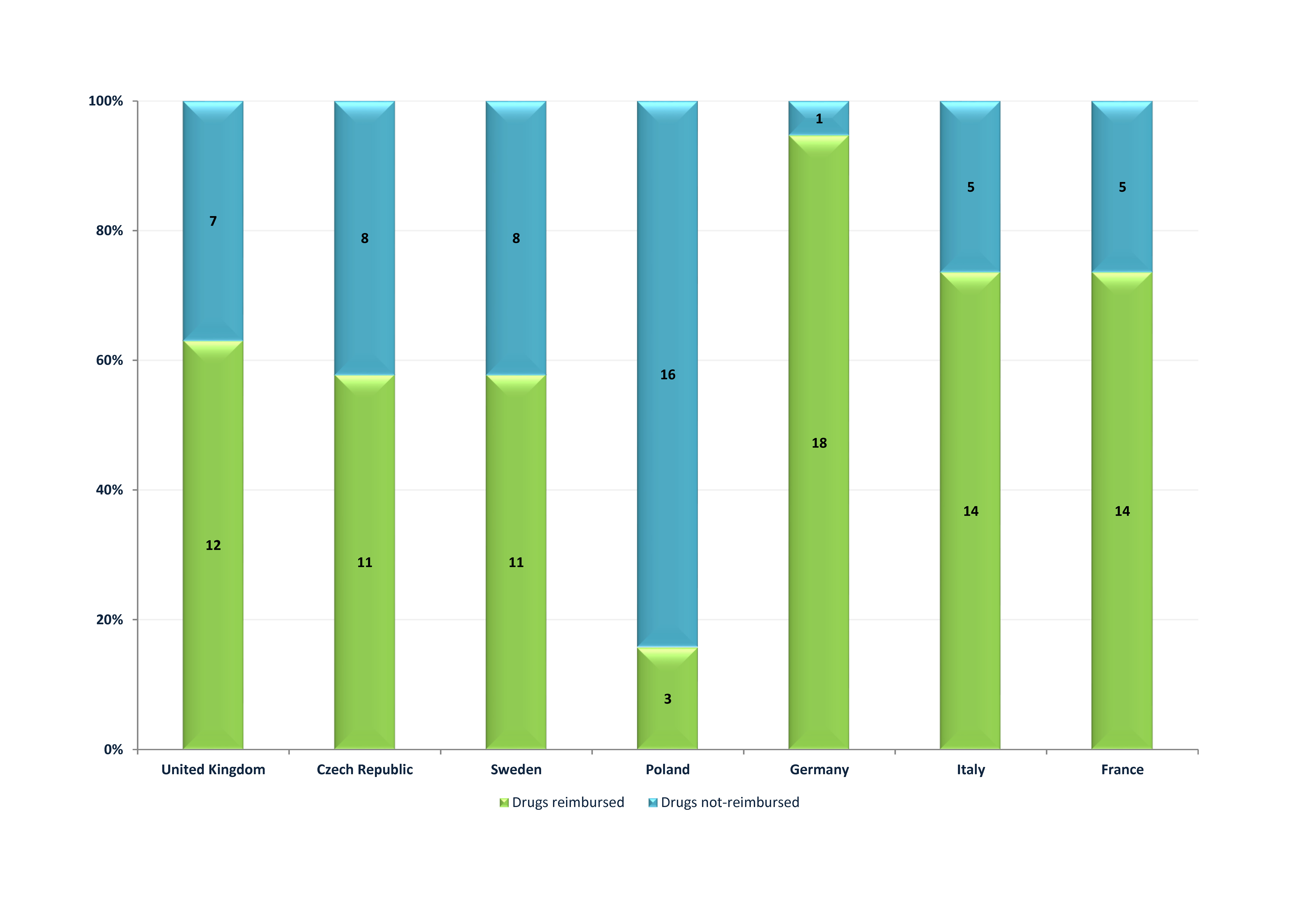
In countries without a cost-effectiveness threshold more new cancer drugs authorised by the European Medicines Agency within the years 2012-2013 have been reimbursed than in countries which use the cost-effectiveness threshold, respectively 80.7% vs. 59.6% (without Poland). If Poland was included, the percentage of reimbursed innovative cancer patient therapies in countries with cost-effectiveness threshold has decreased to an average of 48.7%.
In Poland only 3 out of 19 drugs included in the analysis were reimbursed (15.8%). The group of 16 non-reimbursed products in Poland included products which are reimbursed in all remaining analysed countries — Perjeta, Xalkori and Xtandi.
The detailed numerical data on the availability of drugs authorised by EMA within the years 2012-2013 in reimbursement systems of the countries under analysis was presented on Figure 3.
Additionally, it may be pointed out that the newest drugs (authorised by EMA in 2014) are not reimbursed in most of the selected countries. The exception is Sweden, where a half of 8 new cancer drugs are reimbursed (data not presented).
5-year relative survival rate, incidence and cost per person depending on the reimbursement model used in analysed countries
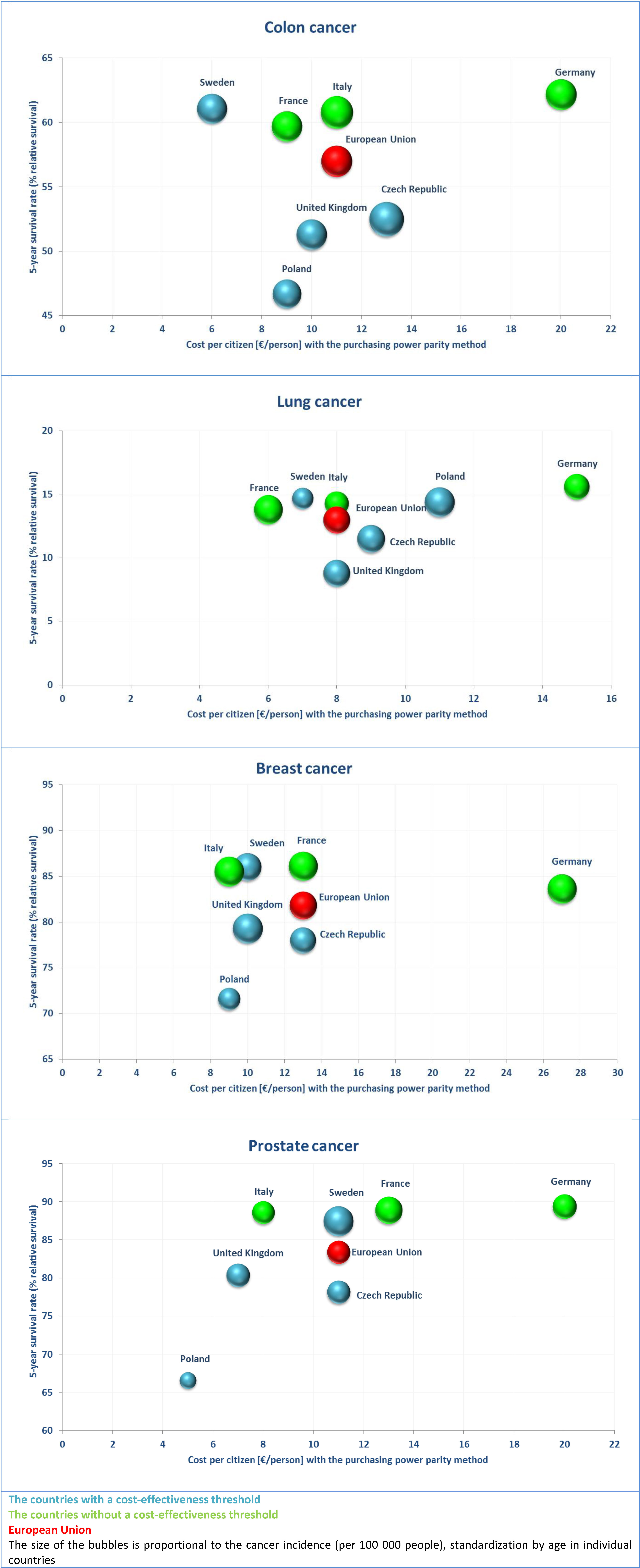
Figure 4 presents epidemiology of and expenses data on most frequently occurring cancers in Poland. Blue was used to mark countries with a cost-effectiveness threshold, green — countries without one. The European Union in total was marked with red.
The data clearly show that in countries without a cost-effectiveness threshold the best health outcomes were achieved, despite differences in expenditure per capita — in Germany it was high, and in remaining countries (Italy and France) was similar to countries with a cost-effectiveness threshold. The 5-year relative survival rates in these countries amounted to approximately 60-62% for colon cancer, 84-86% for breast cancer and approximately 89% for prostate cancer. As a comparison, in most countries which use the cost-effectiveness threshold the values of 5-year relative survival rate in these cancers amounted to 47-53%, 72-79% and 67-80%, respectively. An exception among the countries with a cost-effectiveness threshold was Sweden, for which the 5-year relative survival rates were similar to the ones achieved in countries without the cost-effectiveness threshold, that is 61% in the colon cancer, 86% in breast cancer and 88% in the prostate cancer. This may be caused by the high cost-effectiveness threshold value and by the social criteria for the reimbursement decisions used in this country. In case of lung cancer in contrast to other cancers the total relative survival was low (9-16%), and the differences between individual countries did not exceed a few percentage points.
When comparing countries with a similar incidence of specific cancer and similar expenditure per capita, a better health outcomes were observed in countries which do not use the cost-effectiveness threshold than in the countries which do have the threshold — for example for the colon cancer the 5-year relative survival rate in Italy was higher than in the United Kingdom (approximately 61% vs. 51%). Another example is Poland, where the treatment of the colon cancer resulted in lower 5-year relative survival rate with a similar costs and incidence compared to France, correspondingly approximately 47% vs. 60%.
Compared to other countries, in Poland the observed 5-year relative survival rate in various types of cancers was relatively low (approximately 47% in colon cancer, 72% in breast cancer, 67% in prostate cancer). It was also observed that in Poland the incidence of individual types of cancers was lower than in the remaining European countries under analysis. Likewise, for most types of cancer lower per capita financial expenditure was found (5-11 € per person compared to 7-27 € per person in other countries).
Discussion
The conducted analysis shows that among the cancer drugs authorised by EMA within years 2012-2013 fewer products are financed by the national budget in countries with a cost-effectiveness threshold than in countries without the threshold. The larger number of reimbursed innovative cancer drugs in analysed countries without the cost-effectiveness threshold, that is, Germany, Italy and France, may be caused by the model of reimbursement decision making, in which the key role is played by the drug’s clinical efficacy. Whereas in Poland, the Czech Republic, the United Kingdom and Sweden the validity for the reimbursement of a given technology is its cost-effectiveness.
Because many innovative drugs, mainly orphan and cancer drugs, significantly exceed the established cost-effectiveness thresholds, some of the countries that use this criterion apply alternative financing paths for oncology therapies. For example, in the United Kingdom in specific cases there is a possibility of significantly increasing the cost-effectiveness threshold or of using Cancer Drug Fund with a separate budget for cancer drugs. Whereas in Sweden one of the factors with impact on the cost-effectiveness threshold is the severity of the disease (based on secondary analysis of reimbursement decisions). However, such proceedings do not fully compensate for the restrictive principle of a cost-effectiveness threshold in these countries — their average percentage of reimbursed products is still much lower than in countries without the cost-effectiveness threshold (respectively 61% vs. 81%).
It should be emphasised that in the group of countries with the cost-effectiveness threshold, the lowest percentage of reimbursed new cancer drugs occurs in Poland. The cost-effectiveness threshold value in our country is the same for all assessed technologies, regardless of the disease or the population size. This leads to situation that for most analysed in this study cancer drugs assessed by AOTMiT, the justification for the negative recommendation was the lack of cost-effectiveness of the therapy (for example: Inlyta, Perjeta, Xalkori, Zaltrap, Adcertis, Cometriq, Xtandi).
In countries without the cost-effectiveness threshold the observed health outcomes are clearly better compared to the countries with the threshold. The only exception is the lung cancer, for which the 5-year relative survival rate in all countries is similar. However, it should be noted, that the lung cancer is frequently diagnosed in the late stage of the disease, which currently precludes a successful cure regardless of the level of cancer care [34, 35]. What is interesting, in Sweden, which is a country with the cost-effectiveness threshold, a 5-year relative survival rate is similar to those in countries without the threshold — an explanation for this situation may be the lack of a fixed threshold value – it varies with the therapeutic area.
It was also established that with similar incidence and expenditures per capita, in countries that do not use the cost-effectiveness threshold a better health outcomes can be observed, e.g. a 5-year relative survival in case of colon cancer in Italy is much higher than in the United Kingdom. This may be caused by a limited access to reimbursed innovative cancer drugs for patients resulting from a fixed cost-effectiveness threshold and indicates an ineffective allocation of available resources.
Poland, when compared to selected countries, is characterised by relatively low incidence rates of the selected cancers (with the exception of lung cancer). Notwithstanding, there is a strong premise to consider Polish cancer incidence data to be underestimated compared to reality. Evidence of this is provided by, among others, the higher values of death caused by cancers (in particular in the oldest age groups) compared to the numbers of patients reported to the National Cancer Registry as diagnosed with cancer [36].
The conducted analysis has some limitations. First, its aim was not the statistical assessment of the relation between the analysed parameters. Another element which decreases the credibility of the assessment is the fact that European cancer treatment costs data was obtained for the year 2009, whereas the epidemiological data was obtained for the period before the year 2012. The conducted analyses also did not include the cancer’s stage at the moment of diagnosis, which is important to assess the effectiveness of the therapy. Moreover, the publication was based on data from seven arbitrarily selected countries, and the reimbursement status of medicinal products included in the analysis may change in the future (the presented data are current as of 15th October 2015).
Conclusions
The percentage of reimbursed innovative cancer drugs is higher in countries without the cost-effectiveness threshold, where better health outcomes are observed. Limited access to innovative therapies resulting from restrictive financial criteria may have a significant impact on health outcomes in cancers treatment.
Acknowledgment
This work was supported by Pfizer Polska.
- Bae YHJ., Mullins D. Do Value Thresholds for Oncology Drugs Differ from Nononcology Drugs? J. Manag. Care. Pharm. 2014; 20: 1086-1092
- National institute for Health and Clinical Excellence. Appraising life-extending, end of life treatments. 2009 [cited 15.10.2015]. Available from: https://www.nice.org.uk/guidance/gid-tag387/resources/appraising-life-extending-end-of-life-treatments-paper2;
- Aitken M. Impact of cost-per-QALY reimbursement criteria on access to cancer drugs. IMS Institute for healthcare informatics. 2014; [cited 15.10.2015]. Available from: https://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/IHII_CPQ_Impact_on_Access_to_Cancer_Drugs.pdf;
- Wojciechowska U., Didkowska J. Zachorowania i zgony na nowotwory złośliwe w Polsce. Krajowy Rejestr Nowotworów, Centrum Onkologii - Instytut im. Marii Skłodowskiej - Curie; [cited 15.10.2015]. Available from: http://onkologia.org.pl/raporty/
- De Angelis R., San M., Coleman MP. et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014; 15:23-34
- GLOBOCAN 2012. Estimated Cancer incidence, mortality and prevalence worldwide in 2012. 2012; [cited 15.10.2015]. Available from: http://globocan.iarc.fr/Default.aspx;
- Luengo-Fernandez R., Leal J., Gray A., Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013; 14:1165-1174
- Rémuzat C., Toumi M., Falissard B. New drug regulations in France: what are the impacts on market access? Part 1 - Overview of new drug regulations in France. Journal of Market Access & Health Policy 2013; 1: 20891
- Pricing and Reimbursement Questions. Conférence Bleue. European Lawyers’ Conference on Pharmaceutical and Health Care Affairs. 2015; [cited 15.10.2015]. Available from: http://www.arthurcox.com/wp-content/uploads/2015/06/Pricing-and-Reimbursement-Questions.pdf;
- Bergmann L., Enzmann H., Broich K. et al. Actual developments in European regulatory and health technology assessment of new cancer drugs: what does this mean for oncology in Europe? Ann. Oncol. 2014; 25: 303-306
- IMS Consulting Group. AMNOG: Seven Learnings for Strategic Market Access Decisions in Germany. 2012; [cited 15.10.2015]. Available from: http://pl.scribd.com/doc/290575228/AMNOG-Seven-Learnings-Strategic-Access-Germany#scribd;
- Van Engen A., Latimer NR., Böhler YB., Holmstrom S. Should we adjust overall survival estimates for treatment switching in oncology? Issue Panels - Session I ISPOR 17th Annual Congress. 2014; [cited 15.10.2015]. Available from: http://www.ispor.org/Event/ProgramList/2014Amsterdam?type=IssuePanel;
- Kudrin A. Reimbursement challenges with cancer immunotherapeutics. Hum. Vaccin. Immunother. 2012; 8: 1326-1334
- Ivandic V. Requirements for benefit assessment in Germany and England - overview and comparison. Health Econ. Rev. 2014; 4: 12
- Available from: http://www.agenziafarmaco.gov.it/it; [cited 15.10.2015]
- Available from: http://www.aotm.gov.pl/www/index.php?id=909; [cited 15.10.2015]
- Ustawa z dnia 12 maja 2011 r. o refundacji leków, środków spożywczych specjalnego przeznaczenia żywieniowego oraz wyrobów medycznych. (Dz.U. 2011 nr 122 poz. 696) Available from: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20111220696;
- Available from: http://www.sukl.eu/folder/1533/display/; [cited 15.10.2015]
- Barnieh L., Manns B., Harris Blom M. et al. A Synthesis of Drug Reimbursement Decision-Making Processes in Organisation for Economic Co-operation and Development Countries. Value Health. 2014; 17:98-108
- Mezerová V. Způsoby hodnocení kvality života z pohledu pacjenta. Chech HTA. 2013; [cited 15.10.2015]. Available from: http://czechhta.cz/wp-content/uploads/2013/03/Kvalita-%C5%BEivota-z-pohledu-pacienta.pdf;
- Epstein D. The use of Comparative Effectiveness Research and Health Technology Assessment in European countries: current situation and prospects for the future. Department of Applied Economics, University of Granada. 2014; [cited 15.10.2015]. Available from: https://www.ugr.es/;
- NICE. Process and methods guides. Guide to the methods of technology appraisal 2013. 2013; [cited 15.10.2015]. Available from: https://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf;
- Value Based Assessment of Drugs . Hauses of Parliament. Parliamentary Office of Science & Technology. 2015. POSTnote no. 487; [cited 15.10.2015]. Available from: http://researchbriefings.parliament.uk/ResearchBriefing/Summary/POST-PN-487;
- Available from: http://www.cancerresearchuk.org/about-cancer/cancers-in-general/cancer-questions/cancer-drugs-fund; [cited 15.10.2015]
- NHS England Standard Operating Procedures: The Cancer Drugs Fund (CDF). Guidance to support operation of the CDF in 2014-15. 2014; [cited 15.10.2015]. Available from: https://www.england.nhs.uk/wp-content/uploads/2014/11/sop-cdf-1114.pdf;
- Paris V., Belloni A. Value in Pharmaceutical Pricing. OECD Health Working Paper No. 63, 2013; [cited 15.10.2015]. Available from: http://apps.who.int/medicinedocs/documents/s21368en/s21368en.pdf;
- The Swedish Pharmaceutical Reimbursement System. Pharmaceutical Benefits Board. 2007; [cited 15.10.2015]. Available from: http://www.tlv.se/Upload/English/ENG-swe-pharma-reimbursement-system.pdf;
- Karpa W. Alternatywna metoda kalkulacji progu efektywności kosztowej w analizie farmakoekonomicznej na przykładzie grupy chorób niedokrwiennych serca. Metody Ilościowe W Badaniach Ekonomicznych 2014; XV: 17-25
- Available from: http://www.who.int/choice/costs/CER_thresholds/en/; [cited 15.10.2015].
- Lis J., Orlewska E., Frey J. Podstawy farmaekonomiki. Przegląd Urologiczny 2004; 3:25
- Garau M., Shah KK., Mason AR., Wang Q., Towse A., Drummond MF. Using QALYs in Cancer. A Review of the Methodological Limitations. Pharmacoeconomics 2011; 29: 673-685
- Gaujoux-Viala C., Rat AC., Guillemin F. et al. Responsiveness of EQ-5D and SF-6D in patients with early arthritis: results from ESPOIR cohort. Ann. Rheum. Dis. 2012; 71:1478-1483
- Czech M. Farmakoekonomika. Ekonomiczna ocena programów zdrowotnych. Oficyna wydawnicza PW, 2004; 63-75
- Available from: http://www.cancerresearchuk.org/about-cancer/type/lung-cancer/treatment/statistics-and-outlook-for-lung-cancer; [cited 15.10.2015]
- Rzyman W.: Rak płuc. Forum Medycyny Rodzinnej.2008; 2(6): 407-420
- Kolbarczyk WP., Gujski M., Brzozowski S., Tytko Z., Ścibek A. Walka z nowotworami i opieka onkologiczna w Polsce wobec wyzwań demograficznych i epidemiologicznych – propozycje rozwiązań. Instytut Ochrony Zdrowia. Warszawa 2015; 15