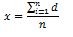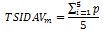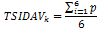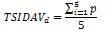Thrombocytopenia Symptoms and their Impact on Patients Daily Activities assessment Vignette (TSIDAV) – validation of the vignette
-
Copyright
© 2016 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Objective: Our aim was to validate the TSIDAV vignette in thrombocytopenia patients.
Methods: The vignettes validation was performed during a study on adults and children with thrombocytopenia. The data were collected among Lublin hospital patients within the period from1st March to 30th April 2016.
Results: We have validated the vignette based on the 61 questionnaires collected among thrombocytopenia patients and children’s caregivers. 31 vignettes were from female patients, 17 from male patients and 13 from children’s caregivers. The most frequently mentioned symptom influencing daily activities in all groups was petechiae and easy bruising. Among men 29% declared it as significant, 68% of women also considered petechiae on their skin and mucosa as having significant impact on their daily activities and 65% declared a significant impact of easy bruising. Among parents and caregivers 62% of them also indicated petechiae as having significant impact on their daily lives and 54% also declared significant easy bruising. We have used a developed special scale for the numerical presentation of the obtained results allowing their interpretation.
Conclusions: The properties of the vignette make it appropriate to assess the impact of the thrombocytopenia symptoms on patients daily activities.
TSIDAV and its dual construction lets us collect the catalogue of different symptoms of thrombocytopenia and their impact of daily life, which could be very helpful in working with thrombocytopenia patients from the psychological and medical point of view.
Introduction
There is a variety of tools to study the QoL, but the instrument that allows collection of two types of data is a vignette. We decided to check whether in such cases it will also be an efficient tool allowing reaching of reliable conclusions. From the psychological point of view the perspective is different, and the vignette is a tool which enables to discover the impact of symptoms on QoL which may contribute to the care of the patient and help doctors become more holistic and prioritize their treatment against the symptoms that most impact their patients’ daily activities. An in-depth analysis of the vignettes used as tools other countries gave us the confidence that thanks to such type of tools will ensure high quality of the results obtained in the study. However, since no such tools exist in Poland yet our vignette should be validated in order to confirm if it is consistent and if it measures what it is intended to measure.
Methods
The validation process consisted of three parts.
The first part consisted of preparation of a tool which would be used to obtain qualitative data on the impact of the most frequent thrombocytopenia symptoms on patients daily activities and quantitative data indicating the intensity with which a symptom of the disease has an impact on the perception on daily activities of patients.
In the second part of the validation process we rated psychometric properties of the new tool: Thrombocytopenia Symptoms and Their Impact on Daily Activities patients assessment Vignette (TSIDAV).
In the third part for the TSIDAV vignette we have developed a scale to determine the impact of thrombocytopenia symptoms on daily activities of patients with thrombocytopenia or their caregivers.
Validation of the vignettes was performed using the guidelines on how to create a tool like the vignette [1].
Vignette validation process followed the following steps:
1. Preparation of principles for creating the vignette;
2. Preparation of a vignette in three versions: for men, women and caregivers of children with thrombocytopenia;
3. The use of vignettes in a defined population (Thrombocytopenia Symptoms and their Impact on patients Daily Activities assessment Vignette -TSIDAV);
4. Assessment of the psychometric properties of the vignettes;
5. Evaluation of the impact of disease symptoms on daily activities of men, women and caregivers of children with thrombocytopenia.
The aim of the first phase of the validation was to develop the guidelines on how to create a tool such as a vignette. The vignette we created aimed at being a tool for qualitative and quantitative research. The guidelines for the construction of a working model of the tool were developed based on the analysis of the tools used in France and the USA [ref].
The next step was to prepare the three versions of vignettes for different populations: women suffering from thrombocytopenia, men with thrombocytopenia and a version for caregivers of children with thrombocytopenia.
Characteristics of the validation group
The data were collected among Lublin hospital patients within the period of 1st March-30th April 2016 among 61 thrombocytopenia patients and children’s caregivers - 31 vignettes were from women, 17 from men and 13 from children caregivers.
The next step was to verify whether the tool can be used to assess the impact of the disease symptoms on the daily activities of people suffering from thrombocytopenia and those who are caregivers of children suffering from thrombocytopenia. Its use can also apply to the qualification process of the symptoms that are perceived by patients and caregivers as more and less burdensome.
The subsequent step in the process of validation of the tool was to preserve the principle of a facade equivalence of maintaining compatibility in terms of graphical representation, the amount of questions and the formulation of questions and the form of response to the questions included, as well as the instructions for research and for selection of the research group.
The vignette for women consists of a descriptive part followed by two questions with the aim to collect qualitative information. The second part included questions related to specific symptoms adjusted for women and included also questions related to menstrual bleeding. The assessment was done using the Likert scale.
The vignette for men contains the descriptive part and two questions aiming at providing qualitative information about the symptoms. The second part included questions related to specific symptoms and the assessment was done using the Likert scale.
The vignette for caregivers of children with thrombocytopenia contains the descriptive part adjusted to children’s activities and two questions aiming at providing qualitative information about the symptoms. The second part included questions related to specific symptoms and the assessment was done using the Likert scale.
The phase 3 of the validation was to develop a special scale used for the numerical presentation of the obtained results and allowing for their interpretation. The vignette includes open-ended questions, to which the patients provide answers without limitations, in an open way. These responses are classified into 5 main domains (Table. 1), which are assigned to values.
Table 1. Domain classification of answers to open questions
|
Domain |
Score |
|
Symptoms associated with thrombocytopenia, related to coagulation |
-2 |
|
Other thrombocytopenia symptoms |
-1 |
|
No symptoms |
0 |
|
Concern, anxiety |
1 |
|
Other diseases symptoms |
2 |
All the responses to the open questions should be classified for each domain. If the answers are contained in a number of domains, then the obtained scores of each domain should be summed up and divided by the number of completed domains according to the formula:
x – value of the question
d – domain value
n – number of completed domains.
According to the above formula it is possible to calculate the value for the questions 1 and 2 of the vignette.
Answers provided in the closed questions, including the Likert scale were assigned to the values.
Next, the score of all the questions must be summed up and divided by the total number of questions included in the vignette, i.e.: vignette for men - 5, for women - 6, for children caregivers - 5. The calculations are made according to the following formulas.
For men:
TSIDAVm – vignette value for men
p – value of the question.
For women:
TSIDAVk – vignette value for women
p – value of the question .
For children – provided by parents or caregivers:
TSIDAVd – vignette value for children – provided by caregivers / parents
p – value of the question
The obtained vignette value may be in a scale between the values of 2 to -2.
The interpretation of the result obtained should be conducted according to the key presented in the Table 2.
Table 2. TSIDAV scale
|
Value |
Interpretation |
|
1.1 – 2.0 |
Very low impact on daily activity |
|
>0 – 1.0 |
Low impact on daily activity |
|
0 |
No impact on daily activity |
|
<0 – -1.0 |
High impact on daily activity |
|
-1.1 – -2.0 |
Very high impact on daily activity |
Results
We have validated the vignette based on the 61 questionnaires collected among thrombocytopenia patients and children’s caregivers. 31 vignettes were from women, 17 from men and 13 from children’s caregivers. The most frequently mentioned symptom influencing daily activities in all groups was petechiae and easy bruising. Among men 29% declared it as significant, 68% of women also considered petechiae on their skin and mucosa as having significant impact on their daily activities and 65% declared a significant impact of easy bruising. Among parents and caregivers 62% also poindicated petechiae as having significant impact and 54% also pointed to significant easy bruising. We have used the specially developed scale for the numerical presentation of the obtained results allowing their interpretation.
The reliability of TSIDAV indicates its semantic and structural similarity to the results described in the epidemiology of thrombocytopenia, which helps to have confidence in vignette as a tool to figure out symptoms of a specific disease and to describe its influence on a daily life of patients.
Conclusions
The properties of the vignette make it appropriate to assess the impact of the thrombocytopenia symptoms on patients’ daily activities.
TSIDAV and its dual construction allows for collection of a catalogue of different symptoms of thrombocytopenia and their impact of daily life, which could be very helpful in working with thrombocytopenia patients from the psychological and medical point of view.
- Szkultecka-Dębek M, Drozd M, BemM, et al. Is the vignette method used to assess quality of life in practice? Curr. Iss. Pharm. Med. Sci. 2015 vol. 28 nr 1 s. 8-12
- Brodowicz M, Zarzycka D. Doniesienie wstępne kulturowej adaptacji i walidacji psychometrycznej Skali Kompetencji Holistycznych Pielęgniarek, Uniwersytet Medyczny w Lublinie, 2015