Budget impact analysis of breast cancer in the Russian Federation
-
Copyright
© 2016 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Breast cancer is one of the leading causes of cancer-related morbidity and mortality in the female population of the Russia and its incidence is rapidly growing. Advanced breast cancer is a disease currently intreatable, but up-to-date management strategies result in significant improvement of symptoms, life expectancy and quality of life. Implementation of new technologies always results in extended healthcare expenditures, therefore requiring pharmacoeconomical justification.
Russian Society for Pharmacoeconomics and Outcomes Research performed health economics evaluation of typical management strategies of patients with advanced hormone-dependent HER2-negative breast cancer, based on survey of experts. The questionnaire developed for this purpose including data relevant to epidemiological and pharmacoeconomic assessment of management of such patients. Calculation of expenses incurred by medications and medical care was performed based on experts’ data and according to the medical care standard #612н. Markov model in Microsoft Excel® application, as developed by York Health Economics Consortium, was used for pharmacoeconomic analysis. The model was based on approaches to treating patients with breast cancer for the duration of 5 years. Managing postmenopausal patients diagnosed with advanced hormone-dependent HER2-negative breast cancer with and without administration everolimus were assessed as alternative approaches, and the method of health economic analysis of budget impact was used to compare them. According to the analysis results, implementation of everolimus administration in the treatment strategies for postmenopausal patients with advanced hormone-dependent HER2-negative breast cancer does not incur significant increases of healthcare budget expenses in the Russian Federation.
Introduction
Breast cancer is the most common malignancies in the female population of economically developed nations. According to the global statistics, every year more than 1.2 million women develop breast cancer, and 450 000 succumb to that disease [1]. In Russia breast cancer ranks as first in the overall structure of cancer incidence and cancer-related mortality in the female population.
In certain prognostically favorable populations of patients with early breast cancer 5-year disease-free survival is 98% [2]. Nevertheless, more than half of breast cancer patients develop remote metastatic lesions. The highest rate of tumor dissemination is at 2-3 years after treatment initiation, although the risk of metastatic spread remains relevant even after 5-10 years after the end of treatment.
Advanced breast cancer is an intreatable disease, but up-to-date treatment approaches have resulted in clinically significant advances in improving both symptoms and life expectancy. Median of overall survival in advanced breast cancer is approximately 24 months [3]. However, in postmenopausal patients with hormone-dependent advanced breast cancer median overall survival can reach 4 years, ranging between 5 and 50 months, even despite multiple metastatic lesions [1].
Pharmacological treatment of advanced breast cancer is aimed at achieving maximum therapeutic effect, improving survival and quality of life. Prognosis is favorable in patients with hormone-dependent cancers, accounting for 70% cases, when beneficial effect of hormonal treatments can be expected [2]. However, hormone medications ultimately result in tumor resistance to such therapy, i.e. treatment failure [3].
Improvement of understanding of the molecular background of cancer pathogenesis has resulted identifying significant number of new targets, correlating with developing new methods of antineoplastic therapy. Development of target agents is focused on specifically affecting certain molecules or receptors in cancer cells, involved in the processes such as cell invasion, metastatic spread, apoptosis, controlling cell cycle and tumor-related angiogenesis.
The PI3K/AKT/mTOR signaling pathway plays a major role in key cellular functions – growth, proliferation. Recent studies show that in in patients with malignancies amplifications, mutations and translocations result in the activation of the PI3K/AKT/mTOR signaling pathway. Activating PI3K mutation has been described in approximately 40% of primary breast tumors, suggesting the importance of PI3K in breast cancer pathogenesis. The mTOR signaling pathway is pivotal in the processes of cell growth, proliferation, regulating apoptosis, angiogenesis and metabolism. Abnormal activation of this pathway by signals passed down from estrogen receptors triggers mechanisms decreasing treatment sensitivity and promoting therapy resistance [4].
One of the latest antineoplastic medications recognized to be beneficial in the treatment of advanced breast cancer is everolimus (Afinitor®) – a selective inhibitor of serine-threonine mTOR kinase. Laboratory experiments have shown that everolimus administration in breast cancer may restore sensitivity of cancer cells to endocrine therapy [5].
Results of a phase 3 randomized multicenter clinical trial (BOLERO-2), including 724 postmenopausal patients with hormone-dependent advanced breast cancer who progressed after treatment with non-steroidal aromatase inhibitors have demonstrated that everolimus, in combination with exemestane, restores sensitivity to endocrine interventions, improving clinical response rate and overall survival [6]. In the group of patients taking everolimus in combination with exemestane, progression-free survival was 6.9 months, as compared to the group primary out-patient facility patients taking exemestane as a single agent (11.0 and 4.1 months, respectively). Objective and clinical response rates in the combination arm were 12.6% and 51.3% whereas in the monotherapy arm they were 1.7% and 26.4%. Safety profile of such combination of two agents proved to be quite acceptable, whereas adverse events were expected and manageable [7].
Therefore, administration of everolimus, a mTOR inhibitor, restores sensitivity of tumor cells to hormonal therapy and increases its antineoplastic activity. Combination of everolimus with exemestane is a novel and effective strategy in postmenopausal patients with hormone-dependent breast cancer resistant to aromatase inhibitors. Everolimus (Afinitor®) provides practical oncologists with new potential options for that will significantly improve treatment outcomes in advanced breast cancer [6,8,9,10]. However, implementation of new technologies always incurs additional healthcare expenses, requiring clinical economical justification.
Therefore, the aim of this study was to perform clinical economics analysis of everolimus administration in the treatment of hormone-dependent HER2-negative advanced breast cancer and its use in postmenopausal patients who progressed after treatment with aromatase inhibitors.
Study objectives:
- To perform analysis and adaptation of the electronic model version provided by the Sponsor for evaluating budget impact of everolimus inclusion in treatment algorithms for hormone-dependent HER2-negative advanced breast cancer in postmenopausal patients.
- To develop a questionnaire for experts dedicated to epidemiological and pharmacoeconomical assessments of managing postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer.
- To perform a survey of experts, from different Russian Federation regions, experienced in managing postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer.
- To adapt the model for evaluating budget implications of everolimus inclusion in treatment algorithms for hormone-dependent HER2-negative advanced breast cancer, with Russian data obtained based on expert survey.
- To evaluate the disease burden associated with breast cancer, when everolimus is included into treatment strategies, as compared to the current treatment strategies in the Russian Federation.
Materials and methods
Evaluating routine practice. Routine practice of managing postmenopausal patients with hormone-dependent HER2-negative (ER+/HER2-) advanced breast cancer was evaluated with a questionnaire containing the following groups of questions: proportion of patients with different breast cancer forms in a given Russian Federation region; medications a their administration schemes in the respective region for the 1st, 2nd and 3rd lines of treatment for postmenopausal patients with ER+/HER2- advanced breast cancer; annual rate of hospitalizations; laboratory and instrumental assessment data, consultation by specialists to which such subjects are referred to on an out-patient basis; adverse events associated with the administration of medications during the treatment of such subjects and the algorithms of their pharmacological management.
Experts who participated in the survey performed their professional activities in the following Russian Federation regions: Moscow, St. Petersburg, Khakassia republic, Omsk region, Primorsky region, Krasnoyarsk region.
Analysis of costs. The medical care standard #612n, approved by the Order of the Ministry of Health of the Russian Federation on November 07, 2012, was used to calculate direct costs incurred by the treatment of one breast cancer patient. When costs incurred by a hospitalization of breast cancer patient were calculated for the period of 10 days, according to the medical care standard, accommodation-related expenses (for hospital stay) were taken into account, along with expenses related to laboratory and instrumental workup, costs of consultations by specialists and medicinal treatments. When costs incurred by medicinal treatments were analyzed, medications administered in more than 30% cases were taken into account. The dose of a chemotherapeutic agent was calculated based on mean body surface area of 1.72 m2.
Costs incurred by medical care were determined using the method developed by Russian Society for Pharmacoeconomics and Outcomes Research according to the medical care prices established by Moscow regional Compulsory Medical Insurance Fund (2013). The proportion of financial contribution from the Compulsory Medical Insurance Fund is approximately 1/3 of the total medical care expenses, which is why the overall sum of medical care expenses for the areas captured by the Compulsory Medical Insurance Fund price establishments were multiplied by 3 [11].
Costs incurred by medicinal products were determined using the average prices as specified in Medline (www.rlsnet.ru) and Pharmindex (www.pharmindex.ru) databases for March-April 2014.
Data relevant to out-patient management of breast cancer were derived from expert survey and included costs incurred by laboratory and instrumental assessments, as well as specialist consultations.
By definition, indirect costs are costs incurred by patient disability or death due to disease or by working losses experienced by caregivers in families. Indirect costs are measured based on working time lost by the patient or her caregivers. However, calculation of such costs requires the data on the friction period – the latter term referring to the time interval after which the employer will fully restore the impaired efficiency (between the first working day lost and up until complete restoration). This value is applicable both to the patient and to her caregivers. Therefore, friction costs constitute a reduction in losses incurred by the patient's being off work, as other employees take over her responsibilities. Such measurements of the friction period and such, consequently, calculations have never been performed in Russia. Therefore indirect costs were not evaluated in this study.
Markov model was used to calculate costs incurred by managing one postmenopausal patient with ER+/HER2- advanced breast cancer on the 1st, 2nd or 3rd lines of treatment. Subsequently the data was extrapolated to analyze the budget implications for the total female population of patients with such disease in the Russian Federation.
Modeling. The following were included into the model:
- The total number of patients of patients with breast cancer in Russia;
- Mean annual increase in the number of breast cancer patients;
- Proportion of patients with advanced breast cancer among breast cancer patients;
- Proportion of postmenopausal patients with ER+/HER2- advanced breast cancer on the 1st, 2nd or 3rd lines of treatment;
- Proportion of postmenopausal patients with ER+/HER2- advanced breast cancer administered everolimus on the 1st, 2nd or 3rd lines of treatment;
- Efficacy of chemotherapy regimens, exemestane and everolimus administration.
Two models of 5-year breast cancer budget implications were quantified during the study; with 1 year duration of each Markov cycle, 5 cycles in total.
Model 1. Treatment of postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer without everolimus.
Model 2. Treatment of postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer with everolimus, if indicated.
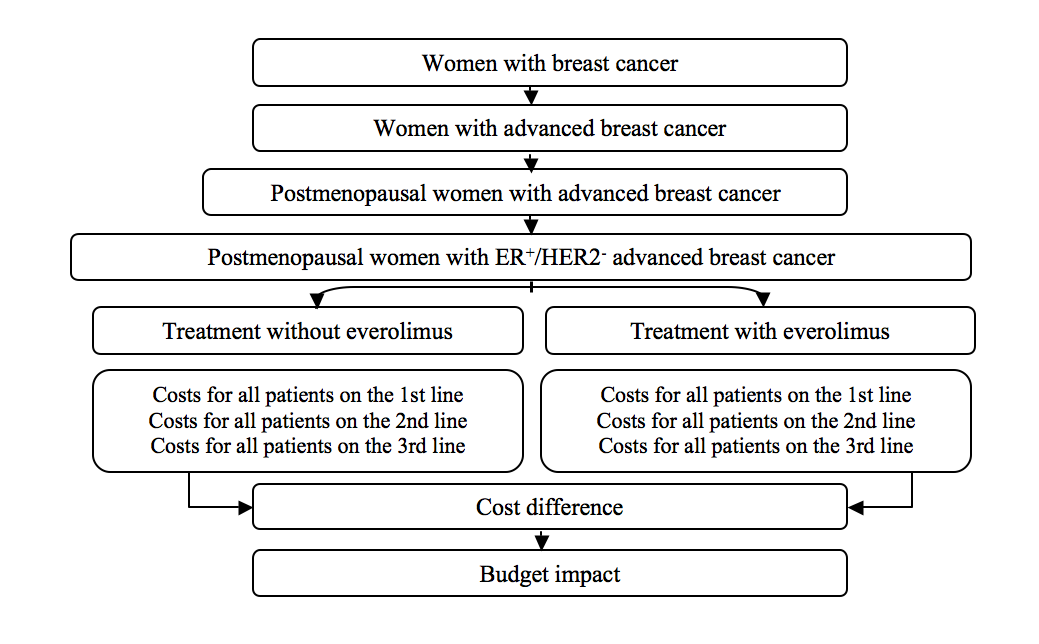
The structure of the analysis investigating budget implications of Models 1 and 2 is presented on Figure 1.
Figure 1. The structure of the model investigating budget implications of everolimus administration in postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer
Study results
Entry of results from two Russian Federation regions has confirmed that the electronic version of the model is valid for investigating budget implications of including everolimus in hormone-dependent HER2-negative breast cancer following progression on aromatase inhibitors in postmenopausal patients. This model is applicable for evaluating budget implications of novel medications in the Russian Federation regions, using data derived from experts in epidemiology and patient management.
The data entered into the model has been derived from the following sources and have the respective values as shown below:
- Data on the total number of breast cancer patients was based on the analysis of malignancy incidence and mortality in the population of the Russian regions as of 2012, taking into account the average annual increase in breast cancer prevalence, resulting in 381.2 cases per 100,000 population [12];
- Data on the mean annual increase in breast cancer prevalence was based on the analysis of malignancy incidence and mortality in the population of the Russian regions as of 2012., resulting in 10,7% [12];
- Data on the proportion of patients with advanced breast cancer was based on the review of oncology care to the Russian Federation population as of 2012, resulting in 9,2 [13];
- Data on the proportion of postmenopausal patients with ER+/HER2- advanced breast cancer receiving 1st line treatment, as provided by expert physicians, resulting in the mean value of 55%;
- Data on the proportion of postmenopausal patients with ER+/HER2- advanced breast cancer receiving 2nd line treatment, as provided by expert physicians, resulting in the mean value of 26%;
- Data on the proportion of postmenopausal patients with ER+/HER2- advanced breast cancer receiving 3rd line treatment, as provided by expert physicians, resulting in the mean value of 19%;
- Data on the proportion of postmenopausal patients with ER+/HER2- advanced breast cancer who were administered everolimus as 1st line treatment, as provided by expert physicians, resulting in the mean value of 2,5%;
- Data on the proportion of postmenopausal patients with ER+/HER2- advanced breast cancer who were administered everolimus as 2nd line treatment, as provided by expert physicians, resulting in the mean value of 3,2%;
- Data on the proportion of postmenopausal patients with ER+/HER2- advanced breast cancer who were administered everolimus as 3rd line treatment, as provided by expert physicians, resulting in the mean value of 4,2%;
- Data on chemotherapy efficacy was obtained from Sjostrom et al. study, (1999) [14];
- Data on exemestane and everolimus efficacy was obtained from the BOLERO-2 study (2013) [15].
The number of breast cancer patients in the Russian Federation as of 2013 was 533,433, of them 49,076 patients had advanced breast cancer. The total number of postmenopausal patients with ER+/HER2- advanced breast cancer was 17,491 cases.
Expert physicians have described treatment strategies applied to postmenopausal patients with ER+/HER2- advanced breast cancer. Results of analysis and summary of expert-provided data are in Table 1.
Table 1. Mean rate of antineoplastic therapy administration for advanced breast cancer
| Treatment regimen | 1st line (%) | 2nd line (%) | 3rd line (%) |
| Letrozol/Anastrozol | 25 | 42,5 | 8 |
| Chemotherapy | 30 | 42,5 | 68 |
| Tamoxifen | 40 | 3 | 0 |
| Fulvestrant | 2,5 | 10 | 21 |
| Avastin + paclitaxel | 2,5 | 2 | 3 |
| Total: | 100% | 100% | 100% |
According to the experts, in average 3% of postmenopausal patients with ER+/HER2- advanced breast cancer receiving 1st or 2nd line of treatment, and 4% postmenopausal patients with ER+/HER2- advanced breast cancer receiving 3rd line of treatment were administered everolimus. According to the experts, the following chemotherapy regimens were used: FAC, CMF, AC, paclitaxel in combination with or without capecitabine, docetaxel, vinorelbin. In average, patients undergo in-hospital treatment 5-6 times a year, according to the administered chemotherapy regimens.
After data entry into the Markov model, the total 5-year costs of managing all breast cancer patients in the Russian Federation have been as follows:
- Without everolimus in treatment strategies for postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer – 75 599 822 707 Russian roubles,
- with everolimus administration – 78 612 254 890 Russian roubles.
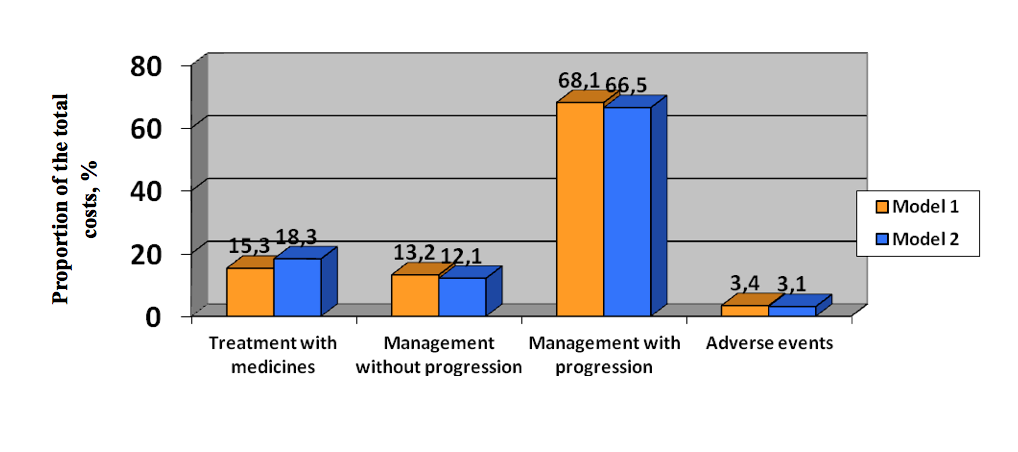
The most significant costs incurred by breast cancer are related to managing patients after disease progression rather than managing complications. Expenses incurred by medicines and treatment till progression are significantly lower than post-progression treatment – both with or without everolimus as part of the treatment strategies for postmenopausal patients with hormone-dependent HER2-negative advanced breast cancer. Only 3% of all patients having indications to everolimus administration were put on treatment regimen containing everolimus, according to the model.
Discussion of results
The main function of the state is to protect citizens’ health. Breast cancer constitutes the area of women's health protection that draws particular attention. Analysis of the disease burden is an essential requisite to plan distribution of healthcare resources, make objective decisions regarding the scope of medical initiatives and determine prices to be used between entities involved in healthcare and medical insurance.
The disease burden associated with breast cancer, according to the results of modeling based on input of 5-year data provided by experts from different regions is consistent with 3.8% increase of costs incurred by this disease if everolimus is used in the treatment strategies for postmenopausal patients with advanced hormone-dependent HER2-negative breast cancer. The medicinal product itself constitutes the largest part of such costs. However, the costs incurred by managing patients and treating complications are reducted when such drug is administered. I.e. treatment with administration of everolimus requires the same costs as treatment without everolimus, if all costs are taken into account, including those incurred by managing complications and significantly advanced tumor forms.
Experts believe everolimus use in managing patients with advanced breast cancer may expand as new data demonstrates its high efficacy in the treatment of bone metastatic lesions.
Summary
- Administration of everolimus in postmenopausal patients with advanced hormone-dependent HER2-negative breast cancer does not result in significant increase of costs, as compared to treatment strategies without such medicinal product. Its inclusion into treatment strategies increases costs by 3.8%, demonstrating negligible implications to the healthcare budget of the Russian Federation. The 5-year disease burden associated with breast cancer was as follows: without including everolimus into the treatment strategies for such patients– 75 599 822 707 Russian roubles, with everolimus – 78 612 254 890 Russian roubles.
- Analysis of the electronic version of the model for evaluating budget implications of everolimus inclusion in treatment strategies for advanced hormone-dependent HER2-breast cancer progressing after treatment with aromatase inhibitors in postmenopausal patients has demonstrated its validity in evaluating budget implications of novel medications.
- The questionnaire developed for the entry of data complication epidemiological and pharmacoeconomical assessment of managing postmenopausal patients with advanced hormone-dependent HER2-negative breast cancer progressing after treatment with aromatase inhibitors ensures input of regional data to the model for its validation.
Conclusion
Therefore, results of the completed study demonstrate that everolimus administration in postmenopausal patients with advanced hormone-dependent HER2-negative breast cancer progressing on/after treatment with aromatase inhibitors, has only negligible implication for the healthcare budget of the Russian Federation.
The article was published with financial support from Novartis Pharma (Russia) according to corporate internal policies and the applicable laws of the Russian Federation. The article contains results of the clinical economics study conducted with support from Novartis Pharma (Russia). Novartis Pharma (Russia) is fully liable for the content of thearticle and its accurate representation of the study results.
- Carrick S, Parker S, Thornton CE et al. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD003372
- National Cancer Institute. SEER Cancer Statistics Review 1975-2008. SEER, 2011. http://seer.cancer.gov/csr/1975_2008/. Accessed October 24, 2011
- Johnston S. New strategies in estrogen receptor-positive breast cancer. Clin. Cancer Res. 2010;16:1979–87
- Semiglazov VF, Dashyan GA, Semiglazov VV, et al. Targetnaya terapiya raka molochnoy zhelezy (novyye napravleniya)// Farmateka, 2011. – №7:p.14-20
- Lane HA, Wood JM, McSheehy PM et al. MTOR inhibitor RAD001 (Everolimus) has antiangio-genic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res 2009;15:1612–22
- Gnant M, et al. Effect of Everolimus on Bone Marker Levels and Progressive Disease in Bone in BOLERO-2 // JNCI (2013), Vol. 105, Issue 9: 654-663
- Yardley DA. Combining mTOR Inhibitors with Chemotherapy and Other Targeted Therapies in Advanced Breast Cancer: Rationale, Clinical Experience, and Future Directions. Breast Cancer: Basic and Clinical Research 2013:7 7–22
- O’Regan R, Ozguroglu M, Andre F, et al. Phase III, randomized, double-blind, placebo-controlled multicenter trial of daily everolimus plus weekly trastuzumab and vinorelbine in trastuzumab-resistant, advanced breast cancer (BOLERO-3) J Clin Oncol. 2013;31(Suppl) Abstr 505
- Treilleux I, Arnedos M, Cropet C, et al. Predictive markers of everolimus efficacy in hormone receptor positive (HR+) metastatic breast cancer (MBC): final results of the TAMRAD trial translational study. J Clin Oncol. 2013;31(Suppl) Abstr 510
- Xie J et al. Budget impact analysis of everolimus for the treatment of hormone receptor positive, human epidermal growth factor receptor-2 negative (HER2-) advanced breast cancer inthe United States // Journal of Medical Economics, Vol. 16, No. 2, 2013, 278–288
- Vorobiev PA, Avksentyeva MV, Borisenko OB, et al. Kliniko-ekonomicheskiy analiz. Edited by P.A.Vorobiev. - M., Publishing house: «Newdiamed». - 2008. – 778 p
- Zlokachestvennyye novoobrazovaniya v Rossii v 2012 godu (zabolevayemost' i smertnost') / Edited by V.I. Chissov, V.V. Starinskyi, G.V. Petrovaya // M.: FGBU «MNIOI named after P.A. Gertsen» of Russian Ministry of Health. – 2012. – il. – 289 sp
- Sostoyaniye onkologicheskoy pomoshchi naseleniyu Rossii v 2012 godu / Edited by A.D. Kaprin, V.V. Starinskyi, G.V. Petrovaya // M.: FGBU «MNIOI named after P.A. Gertsen» of Russian Ministry of Health, 2013. – il. – 232 p
- Sjostrom J, Blomqvist C, Mouridsen H, Pluzanska A, Ottosson-Lonn S, Bengtsson NO. Docetaxel compared with sequential methotrexate and 5-fluorouracil in patients with advanced. European Journal of Cancer 1999;35(8):1194–1201
- Burris HA, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the Phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915