Treatment and prevention of bleeding in adult hemophilia A patients with inhibitor – economic analysis
-
Copyright
© 2016 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Hemophilia A is caused by an absence or deficiency of coagulation factor VIII. Patients with hemophilia may experience recurrent spontaneous hemarthroses or internal bleeding. Following the treatment with factor VIII concentrates, patients with hemophilia A may develop alloantibodies to factor VIII, evidence of which is critical to diagnose hemophilia with inhibitor. The primary goal of treatment in patients with hemophilia with inhibitor is a durable inhibitor elimination and the interim goal is to stop the bleeding. The aim of this paper is to compare the effectiveness and costs of on-demand therapy and prophylaxis in patients with hemophilia A with or without inhibitor. We conducted a review of studies. The outcomes of the studies included in the review suggested that the difference in annual bleeding rate (ABR) between prophylaxis and on-demand therapy is less pronounced in patients with inhibitor. Furthermore, one study found no statistically significant difference in ABR between prophylaxis and on-demand therapy in patients aged ≥ 40, although the consumption of coagulation factor was significantly higher in the prophylaxis group. Treatment of patients with hemophilia A is associated with high costs of coagulation factor concentrates and frequent, stressful and painful injections. Therefore, while considering the introduction of prophylaxis in adult patients, it appears advisable to select groups of patients depending on the frequency of bleeding episodes and to determine adequate treatment strategy.
Introduction
Hemophilia A is a disorder caused by an absence or deficiency of coagulation factor VIII (FVIII). Depending on the coagulation factor VIII level, hemophilia is defined as severe (<1% of normal factor level, 0.01 IU/ml), moderate (1%-5% of normal factor level, 0.01-0.05 IU/ml) or mild (5%-50% of normal factor level, >0.05-<0.50 IU/ml) [1]. As a consequence of treatment with factor VIII concentrates, patients with hemophilia A may develop alloantibodies to factor VIII, evidence of which is critical to diagnose hemophilia with inhibitor. Approximately 15-30% of patients with severe hemophilia develop factor VIII inhibitor [2].
In accordance with the Polish National Health Program for Patient with Hemophilia and Bleeding Diatheses (2012-2018) (Narodowy Program Leczenia Chorych na Hemofilię I Pokrewne Skazy Krwotoczne na lata 2012-2018) 2,263 patients (adults and children) were registered by Institute of Hematology and Transfusion Medicine in Warsaw, Poland (Instytut Hematologii i Transfuzjologii w Warszawie) by 17th of September 2013, including 1,071, 331 and 713 with severe, moderate and mild hemophilia, respectively [2].
Recurrent spontaneous hemarthrosis is the major symptom of severe hemophilia. Hemarthrosis results in arthropathy leading to significant decrease in physical activity and even early labor market exit. Patients with hemophilia may also develop severe and life-threatening spontaneous bleeding to internal organs and body cavities (e.g. intracerebral hemorrhage or gastrointestinal bleeding) or excessive bleeding after trauma [2].
Management of patients with hemophilia A
The mainstay of treatment for severe hemophilia A is factor VIII replacement therapy, administered as [2]:
a) on-demand therapy – factor concentrate injections given for clinically evident bleeding episodes;
b) prophylaxis:
- primary prophylaxis – regular injections of factor concentrates initiated before documented arthropathy has occurred and after second, clinically significant episode of large joint bleed in patients before the age of 3 years to prevent arthropathy;
- secondary prophylaxis – regular factor concentrate injections started after 2 or more bleeds into joint/joints and before arthropathy has occurred;
- tertiary prophylaxis – regular factor concentrate injections initiated after arthropathy has occurred;
- short-term prophylaxis – regular factor concentrate injections, for less than 45 weeks per year, in patients with hemophilic arthropathy to stop recurrent bleeding into a particular joint or to prevent bleeding during physiotherapy;
- perioperative prophylaxis – factor concentrate injections started prior to surgery and continued until healing is achieved to prevent bleeding in the perioperative period [1].
The development of inhibitor to FVIII is considered to be severe complication in patient with hemophilia, as coagulation factors administered as replacement therapy become inactive. The primary aim of treatment in hemophilia patients with inhibitor is a durable elimination of the inhibitor and prevention of bleeding. The therapeutic strategy to eliminate inhibitors is to administrate regular injections of factor VIII concentrates (immune tolerance therapy) [2]. Dosing frequency in immune tolerance induction is varied, starting with frequent and regular doses and ending with protocols involving significantly higher doses [3]. In order to control bleeding episodes, bypassing agents, inducing thrombin generation in plasma, are used despite the presence of inhibitor to FVIII. Currently, two bypassing agents are used, i.e.: activated prothrombin complex concentrates (aPCC, Feiba®) and recombinant activated factor VII (rFVIIa, NovoSeven®) [1].
In 2008 a therapeutic program for bleeding prophylaxis in children was implemented in Poland (Prevention of bleeding in pediatric patients with hemophilia A and B). The program is reimbursed by Polish National Health Fund. First therapeutic program for adults (Program for hemophilia and bleeding diatheses treatment with coagulation factors), reimbursed by the Ministry of Health,was implemented in 2001. The current treatment program for the years 2012-2018 is a continuation of the program for the years 2005-2011 [1]. The program provides on-demand therapy and short-term prophylaxis (a few months or weeks) in adult patients with recurrent bleeding into a particular joint or muscle and who are not eligible to primary or secondary prophylaxis [4]. The aim of this article is to review current clinical strategies for treatment of hemophilia in adults.
Results
Prophylaxis vs on-demand therapy in patients aged 40 years or older
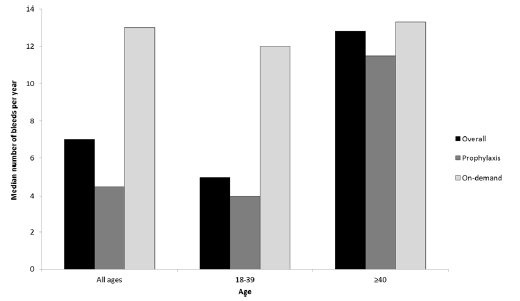
In the clinical trials on patients with hemophilia A without inhibitor, compared to on-demand therapy, prophylaxis was associated with a significant reduction in the frequency of bleeding episodes (including joint bleeds), however, the difference between prophylaxis and on-demand therapy is less pronounced in hemophilia patients with inhibitor [5, 6]. In addition, in the clinical trials comparing prophylaxis and on-demand therapy, most patients were children and young adults (aged ≤ 40 years old) [1]. Jackson et al. (Jackson 2015) conducted one of the few studies in patients aged 40 years or older [7]. In this observational study prophylaxis was compared with on-demand therapy of severe hemophilia A in patient aged 40 years or older and younger patients. The study included 220 adult patients from Canada, with 70% of patients being exposed to prophylaxis and 27% aged 40 years or older. Hemophilia with inhibitor affected about 15.6% and 35% of younger and older patients, respectively. Annualized bleeding rate (ABR) was considered to be the primary endpoint in the study. Jackson et al. (Jackson 2015) revealed statistically significant differences between prophylaxis and on-demand therapy in terms of ABR (4 vs. 12 bleeding episodes/year; p<0.0001) in hemophilia A patients with our without inhibitor, who were aged ≤ 40 years old (Figure 1). There were no statistical differences in older subjects, aged ≥ 40 years old (12 vs. 13 bleeding episodes/year; p=0.866), even though the discrepancy between factor utilization for on-demand therapy versus prophylaxis was observed (560 vs. 3447 u/kg/year, p < 0.001). We sent and inquiry to the authors of the study on the difference between prophylaxis and on-demand therapy with regard to ABR in patients with hemophilia A with inhibitor, but no response has been received. In all patient aged 40 years or older hemophilia A with inhibitor affected about 35% of patients. Therefore, it is likely that in this age group no significant differences between prophylaxis and on-demand therapy in ABR are present both, in patients with and without inhibitors.
Source: Jackson et al.2015 [7]
Comparison of prophylaxis and on-demand therapy for adult patients with hemophilia A with inhibitor
Currently, there are no clear guidelines for the use of prophylaxis in adult patients with hemophilia [4]. These uncertainties are related to the high costs of prophylaxis in patients with arthropathy due to the lack of prophylaxis in childhood and adolescence. The benefits of prophylaxis in this group of patients are limited to reduced bleeding rate, while the influence on inhibition of arthropathy progression is unclear. Scientific evidence shows that secondary prophylaxis in adult patients with hemophilia A aged ≥ 40 years old is ineffective. Hence, the cost of prophylaxis and on-demand therapy in the group of patients aged ≥ 40 years old with hemophilia A with the inhibitor were compared. Data were based on the study Jackson et al., 2015 [7]. The estimates used median annual number of bleeding episodes in relevant age subgroups.
Two replacement therapies in the prevention of bleeding episodes for patients with hemophilia A with inhibitor are available in Poland: i.e.: NovoSeven® and Feiba® [8,9]. The products are considered to have comparable clinical efficacy [10]. NovoSeven®, according to the product characteristics, is indicated only for the treatment of bleeding and for the prevention of bleeding in patients undergoing surgery or invasive procedures (home therapy available on request only) [11]. Feiba® is indicated for the treatment and prevention of bleeding (on-demand therapy and prophylaxis) [12]. Considering the comparable efficacy of on-demand therapy and prophylaxis, it is assumed that the only differing cost is associated with the various consumption of coagulation factors.
Prices of coagulation factors were estimated based on Polish National Blood Center data [8] and data from tenders announced by Department of Public Procurement at the Ministry of Health [14]. The Table 1 and Table 2 present estimated prices of one microgram (for NovoSeven®) and a single unit (for Feiba®).
Table 1. rFVIIa consumption and expenditure in treatment of hemophilia patients with inhibitor based on data of National Blood Center [14] and Department of Public Procurement at the Ministry of Health [8]
|
Source |
Consumption rFVIIA (µg) |
Expenditure (PLN) |
|
Tender ZZP-38/14 |
13 000 000.00 |
36 920 000.01 |
|
Tender ZZP-159/15 |
1 800 000.00 |
5 112 000.00 |
|
Tender ZZP-125/15 |
10 300 000.00 |
29 252 000.00 |
|
Tender ZZP-90/15 |
11 000 000.00 |
31 240 000.00 |
|
National Blood Center data from year 2013 |
13 559 000.00 |
38 507 560.00 |
|
Total |
49 659 000.00 |
141 031 560.01 |
|
Cost per unit (PLN/µg) |
2.84 |
|
Table 2. aPCC consumption and expenditure in treatment of hemophilia patients with inhibitor based on data of National Blood Center [14] and Department of Public Procurement at the Ministry of Health [8]
|
Source |
Consumption aPCC (unit) |
Expenditure (PLN) |
|
Tender ZZP-130/15 |
6 000 000.00 |
22 740 000.00 |
|
Tender ZZP-89/15 |
10 000 000.00 |
37 900 000.00 |
|
Tender ZZP-157/15 |
5 600 000.00 |
21 224 000.00 |
|
Tender ZZP- 155/14 |
10 000 000.00 |
37 900 000.00 |
|
Tender ZZP-121/14 |
1 400 000.00 |
5 306 000.00 |
|
Tender ZZP-36/14 |
6 000 000.00 |
22 740 000.00 |
|
National Blood Center data from year 2013 |
8 498 000.00 |
32 207 420.00 |
|
Total |
47 498 000.00 |
180 017 420.00 |
|
Cost per unit (PLN/unit) |
3.79 |
|
On-demand therapy cost
The treatment costs of a single bleeding episode were estimated taking into account prices depicted in Table 1 and Table 2. The drug doses necessary to control a bleeding episode were adopted on the basis of data reported by Goszczyńska et al. 2011 [9] and Lyseng‑Williamson and Plosker 2007 [13]. Lyseng‑Williamson and Plosker summarized data from the following publications: Dundar et al. 2005 [15], Hart 2002 [16], Odeyemi and Guest 2002 [17], Ozelo et al. 2007 [18], Plyush et al. 2006 [19], and Yoo et al. 2007 [20]. The authors of these papers report drug doses used in the general population of hemophilia patients with inhibitor. Estimates of the doses and the costs of Feiba® or NovoSeven® in the treatment of a single bleeding episode are presented in the Table 3. Differences in costs of single bleeding episode treatment with Feiba® and NovoSeven® are about PLN 10 000 in favor of NovoSeven®. Both therapies have similar number of doses required to control a bleeding episode.
Table 3. Costs and drug doses utilized to control single bleeding episode in population of hemophilia patients with inhibitor
| Dundar et al. 2005 | Hart 2002 | Odeyemi and Guest 2002 | Ozelo et al. 2007 | Plyush et al. 2006 | Yoo et al. 2007 | Goszczyńska et al. 2011 | Mean | ||
| Country | Turkey | Slovakia | Great Britain | Brazil | Russia | South Korea | Poland | na | |
| Mean umber of injections administrated in order to control a bleeding episode | rFVIIa | 3.60 | 2.10 | 2.30 | 2.00 | 1.60 | 1.70 | nd | 2.22 |
| aPCC | 4.80 | 2.00 | 3.00 | 3.80 | 1.70 | 2.30 | nd | 2.93 | |
| Mean dose required to control a bleeding episode | rFVIIa (ug/kg bw) |
204.00 | 160.00 | 207.00 | 190.00 | 157.00 | 136.00 | 219.00 | 181.86 |
| aPCC (unit/kg bw) | 167,00 | 105.00 | 225.00 | 260.00 | 135.00 | 168.00 | 176.00 | 176.57 | |
| Cost of a single bleeding episode treatment from public payer perspective* | rFVIIa (PLN) | 42 009.39 | 32 948.54 | 42 627.18 | 39 126.40 | 32 330.76 | 28 006.26 | 45 098.32 | 37 449.55 |
| aPCC (PLN) | 45 893.75 | 28 855.35 | 61 832.90 | 71 451.35 | 37 099.74 | 46 168.57 | 48 367.07 | 48 524.11 | |
bw – body mass, nd – no data, na – not applicable
*taking into account unit price from table 1 and table 2(determined on the basis of National Blood Center data and data from tenders announced by Department of Public Procurement at the Ministry of Health [14]), and assuming a mean body mass of 72.51 kg [21]
Jackson et al. [7] presented results referring to hemophilia patients (with and without inhibitor). Patients aged ≥ 40 years old on prophylaxis had an ABR of 12, while those on-demand use had an ABR of 13. Adult patients aged ≥ 40 years old and on-demand use had an ABR of 12. The estimates of annual costs of on-demand therapy in patients aged ≥ 40 years are depicted in the Table 4.
Table 4. Estimates of annual costs of on-demand therapy for single hemophilia patient with inhibitor
| On-demand therapy using aPCC only | On-demand therapy using rFVIIa only | |
| ABR in subgroup of patients aged ≥ 40years | 13.00 | 13.00 |
| Cost of on-demand therapy (PLN/year) | 630 813.38 | 486 844.16 |
*assuming mean patient body weight of 72.51 kg [21]
Cost of prophylaxis
While estimating the annual cost of prophylaxis, doses recommended by the Medical and Scientific Advisory Council (MASAC) [22] were used. Considering the therapeutic indications, it was assumed that in long-term prophylaxis only Feiba® will be used [11,12]. Estimation of the annual cost of prophylaxis made on the basis of MASAC 2013 guidelines is summarized in the Table 5. The recommended dose of Feiba® in the prophylaxis of bleeding is slightly lower in the MASAC 2013 guidelines (three times a week) than in the product characteristics (every other day). However, the dose is still within the range of the recommended dose adjustments and it seems to be in line with everyday clinical practice. Additionally, we performed a non-systematic review of the literature to identify publications reporting prophylactic aPCC consumption in practice. Negrier et al. reported significant differences in practical aPCC dosing in prophylaxis [23]. It is associated with the need for an individual dose adjustment. These values and drug costs are summarized in the Table 6. Given the wide range of doses used in practice, it was assumed that the average annual consumption of Feiba® is equivalent to its consumption determined on the basis of the MASAC 2013 guidelines. It should be noted that in Jackson 2015 publication, patients aged ≥ 40 years old on prophylaxis were administered higher doses than younger patients. Therefore, it can be assumed that the consumption of coagulation factor (and the cost of prophylaxis) among older patients is higher than the average consumption determined on the basis of MASAC 2013. The Table 7 summarizes the costs of prophylaxis and on-demand therapy, which will be generated by patients aged ≥ 40 years old on prophylaxis. ABR was adopted on the basis of Jackson 2015 publication. Given the fact that Jackson 2015 et al. proved no statistically significant differences in the ABR between patients aged ≥ 40 years old on prophylaxis or on-demand therapy, quality of life of these patients may be reduced on prophylaxis due to the frequent dosing (quality of life decrease associated with injections). Prophylactic injections are usually given at least three times a week. Matza 2013 et al. [24] presented the influence of injections and infusions on the quality of life in patients suffering from bone metastases. Basing on these results, we assumed that the loss on quality of life due to injections and infusions is comparable in hemophilia patients and in patients with bone metastases. A single injection is associated with a decrease in the patients’ quality of life by 0.4%, while the half-hour infusion by 2.3%. Prophylaxis does not guarantee an improvement in general condition in patients aged ≥ 40 years old, and additionally, frequent injections or infusions may be an important factor decreasing the quality of life. The decision whether to administer prophylaxis may be influenced by the difficulties concerning this treatment. This fact may be of particular importance in patients with hemophilia with inhibitor, who tend to receive on-demand therapy [7].
Table 5. Recommended aPCC dosage and costs of long-term prophylaxis per one patient
| Dosage based on MASAC 2013 | Weekly dosage | Annual cost of drug (PLN) | |
| aPCC | 85 unit/kg bw 3 times a week | 255.00 unit/kg bw | 3 656 532.86 |
Table 6. Dosage and cost of prophylaxis per one patient
| Min. weekly dosage | Max. weekly dosage | Annual cost – minimum variant (PLN) | Annual cost – maximum variant (PLN) | |
| aPCC (Negrier 2016) |
30.61 unit/kg bw | 1 075.20 unit/kg bw | 438 906.43 | 15 417 663.24 |
Table 7. ABR and annual costs of prophylaxis and on-demand therapy of single patient aged above ≥40 years on prophylaxis with Feiba®
|
Value |
|
|
ABR in the age group aged ≥ 40 years |
12.00 |
|
Cost of on-demand therapy (PLN per year) |
582 289.28 |
|
Cost of prophylaxis (PLN per year) |
3 656 532.86 |
|
Total cost (PLN per year) |
4 238 822.13 |
Discussion and Conclusions
Patients with hemophilia and related bleeding diatheses represent only a limited part of the general population. However, taking into account the frequent hospitalization need, very high cost of treatment and the difficulties of rehabilitation, it can be stated that hemophilia is a social issue [2]. Hemophilia therapy is associated with high costs of coagulation factor concentrates and frequent, stressful and painful injections [4]. Moreover it often leads to permanent disability which has a huge economic impact on families and the entire society. According to Forsyth et all optimal bleeding control therapy decreases pain, prevents further disability and results in better quality of life [25]. We conclude that on-demand treatment is equally effective as prophylaxis in patients aged ≥ 40 years. The Table 8 presents a summary of treatment costs, proving that prophylaxis in hemophilia patients, aged ≥40 years, experiencing an average ABR, compared to on-demand treatment, is associated with several times higher costs. Extrapolation of this age group results from Jackson 2015 on hemophilia with inhibitor patients allows to conclude that higher costs do not cause a significant improvement of the health state (in patients aged ≥ 40 years, ABR was comparable both on prophylaxis and on-demand therapy). Older subjects with prophylaxis had a higher ABR than younger subjects (12 vs 4). The authors did not provide explanation for ABR age-related differences.
Table 8. Summary of costs depending on treatment option in patients aged ≥ 40 years
| Cost of single patient prophylaxis with aPCC | Cost of single patient on demand treatment with aPCC | Cost of single patient on demand treatment with rFVIIa | |
| ABR in patients aged ≥ 40 years | 12.00 | 13.00 | 13.00 |
| Cost of acute treatment (PLN per year) | 582 289.28 | 630 813.38 | 486 844.16 |
| Cost of prophylaxis (PLN per year) | 3 656 532.86 | - | - |
| Total cost (PLN per year) | 4 238 822.13 | 630 813.38 | 486 844.16 |
On-demand therapy with NovoSeven® is cheaper than treatment with Feiba®. The difference in the treatment cost for single bleeding episode is about PLN 10 000. It should be noted that prophylaxis, despite its significantly higher cost, does not provide significant improvement for patients aged ≥ 40 years and inconveniences associated with frequent injections (several times a week) may have the opposite effect to that which is intended. Therefore, while considering the introduction of prophylaxis in adult patients, it appears advisable to select groups of patients depending on the frequency of bleeding episodes and to determine adequate treatment strategy (long-term prophylaxis, short-term prophylaxis, indefinite prophylaxis and on-demand therapy) [4].
- Windyga J. Hemofilie – postępy w diagnostyce i leczeniu. Acta Haematologica Polonica 2010; 41 (2): 183–199
- Ministry of Health. Narodowy Program Leczenia Chorych na Hemofilię i Pokrewne Skazy Krwotoczne na lata 2012-2018. 2012; Available from: http://www.mz.gov.pl/zdrowie-i-profilaktyka/krwiodawstwo/program-zdrowotny-narodowy-program-leczenia-chorych-na-hemofilie-i-pokrewne-skazy-krwotoczne-na-lata-2012-2018/; [Accessed: 11.06.2016]
- Windyga J, Chojnowski K, Klukowska A. et al. Polskie zalecenia postępowania we wrodzonych skazach krwotocznych na tle niedoboru czynników krzepnięcia. Acta Haematologica Polonica 2008; 39 (3): 565–579
- Jamrozik M, Krawczyk-Kuliś M, Kyrcz-Krzemień S. et al. Rola profilaktyki okresowej w leczeniu ciężkiej i umiarkowanej hemofilii. Katedra i Klinika Hematologii i Transplantacji Szpiku Śląskiego Uniwersytetu Medycznego 2015; 1-12
- Blanchette VP. Prophylaxis in the haemophilia population. Haemophilia 2010; 16 (5): 181–188
- Leissinger C, Gringeri A, Antmen B, et al. Anti-Inhibitor Coagulant Complex Prophylaxis in Hemophilia with Inhibitors. NEJM 2011; 365: 1684-92
- Jackson SC, Yang M, Minuk L, et al. Prophylaxis in older Canadian adults with hemophilia A: lessons and more questions. BMC Hematology 2015; 15 (4): 1-8
- Narodowe Centrum Krwi, Wyniki raportu za 2013 rok sporządzone w oparciu o dane przesłane przez wojewódzkich koordynatorów ds. leczenia hemofilii i pokrewnych skaz krwotocznych. 2013 [Accessed: 11.06.2016]. Available from: http://www.nck.gov.pl/wp-content/uploads/2014/06/Wyniki-raportu-Jednostki-Koordynuj%C4%85cej-za-2013-rok.pdf
- Goszczyńska K, Wrona W, Niewada M. Porównanie kosztów terapii lekami omijającymi inhibitor w leczeniu łagodnych i umiarkowanych krwawień u chorych z hemofilią wrodzoną powikłaną inhibitorem w Polsce. Journal of Transfusion Medicine 2011; 4 (3): 115-122
- Franchini M, Coppola A, Tagliaferri A, Lippi G. FEIBA versus NovoSeven in Hemophilia Patients with Inhibitors. Seminars in Thrombosis & Hemostasis 2013; 39 (7): 772-778
- European Medicines Agency, Charakterystyka Produktu Leczniczego NovoSeven® 2016. [Accessed: 11.06.2016]. Available from: http://www.ema.europa.eu/docs/pl_PL/document_library/EPAR_-_Product_Information/human/000074/WC500030873.pdf
- Baxter, Charakterystyka Produktu Leczniczego Feiba® 2010. [Accessed: 11.06.2016]. Available from: http://www.baxter.com.pl/downloads/charakterystyki/BioScience/Feiba_NF.pdf
- Lyseng-Williamson KA, Plosker GL. Recombinant Factor VIIa (Eptacog Alfa). A Pharmacoeconomic Review of its Use in Haemophilia in Patients with Inhibitors to Clotting Factors VIII or IX. Pharmacoeconomics 2007; 25 (12): 1007-1029
- Zakład Zamówień Publicznych przy Ministrze Zdrowia, Zużycie i wydatki na preparat rFVIIa stosowany w leczeniu chorych na hemofilię powikłaną inhibitorem na podstawie przetargów 2013 [Accessed: 11.06.2016]. Available from: http://www.zzpprzymz.pl/ogloszenia-o-przetargach.php
- Dundar S, Zülfikar B, Kavakli K, et al. A cost evaluation of treatment alternatives in mild-to-moderate bleeding episodes in haemophilia patients with inhibitors in Turkey. J Med Econ 2005; 8: 45-54
- Hart WM. An economic evaluation of NovoSeven in the man agement of haemophilia patients with inhibitors in Slovakia [abstract no. POD1, poster presented at the 5th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Resear]. Value Health 2002; 5 (6): 576- 7
- Odeyemi AO, Guest JF. Modelling the economic impact of recombinant activated factor VII compared to activated pro- thrombin-complex concentrate in the home treatment of a mild to moderate bleed in adults with inhibitors to clotting factors VIII and IX in the UK. J Med Econ 2002; 5: 119-33
- Ozelo MC, Villaca PR, De Almeida JO, et al. A cost evaluation of treatment alternatives for mild-to-moderate bleeding episodes in patients with haemophilia and inhibitors in Brazil. Haemophilia 2007; 13 (5): 462-9
- Plyush O, Kopylov K, Zozulya N, et al. A cost evaluation of treament alternatives in mild-to-moderate bleeding episodes in haemeophilia patients with inhibitors in Russia [in Russian]. Paediatr Haematol Oncol Immunopathol 2006; 5 (3): 16-22
- Yoo SK, Yong LS, Kyu PS, et al. Pharmaco-economic evaluation of treatment alternatives for mild to moderate bleeding episodes in patients with haemophilia with inhibitors in Korea [abstract no. P-W-158 plus poster]. 21st Congress of the Inter- national Society on Thrombosis and Haemostasis 2007
- Estymator, Wyniki badań ankietowych na temat: waga i nadwaga Polaków, wrzesień 2006, http://www.estymator.com.pl/
- National Hemophilia Foundation, Masac Recommendation Regarding Prophylaxis With Bypassing Agents In Patients With Hemophilia And High Titer Inhibitors 2013. [Accessed: 11.06.2016]. Available from: https://www.hemophilia.org/sites/default/files/document/files/masac220.pdf
- Negrier C, Voisin S, Baghaei F et al. Global Post-Authorization Safety Surveillance Study: real-world data on prophylaxis and on-demand treatment using FEIBA (an activated prothrombin complex concentrate). Blood Coagul Fibrinolysis 2016; 27 (5): 551-6
- Matza L, Cong Z, Chung K. et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Preference and Adherence 2013; 7: 855–865
- Forsyth A, Witkop M, et al. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study, Patient Prefer Adherence. 2015; 9: 1549–1560