The disposal of unused and/or out of date medications in the community pharmacy setting in Poland – a challenge for pharmaceutical public health and pharmaceutical care
-
Copyright
© 2016 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
INTRODUCTION
In Poland, the availability of medicinal products is quite high, despite a temporary shortage of certain drugs, particularly antiplatelet and anticoagulant agents [1]. This is vastly different from the situation of Communist-era Poland, when access to then-modern drugs was severely limited due to issues related to intellectual properties, and limitations in transferring medications from Western to Eastern Europe. In the past 30 years, the number of community pharmacies has drastically increased, and Poland boasts one of the highest numbers of community pharmacies per patient in Europe [2]. Recent amendments to the pharmaceutical law introduced a list of medicinal products available free of charge for patients over 75 years of age. This increase in accessibility to medications among the elderly may prove beneficial in improving adherence [3]. All of these facts draw attention to a new issue emerging in Poland - the disposal of unused and/or outdated medications in light of protecting our natural environment [4]. This problem is not only purely theoretical but has a direct impact on workload in the community pharmacy. Pharmacists should bear in mind that medical waste remains particularly dangerous due to the possibility of HIV or HCV infection after unintended injury [5]. The problem of unused and/or outdated medicinal products in Poland has many factors. It is related to drugs transferred for patient utilization, those drugs left unused by the patient, and self-producing waste from the community pharmacy. In this instance, some of the drugs may be already expired before purchased by the patients or unused before compounding the formulation. Accordingly, these issues will be discussed in further parts of this paper.
Currently, there are two main ways of transferring an active pharmaceutical ingredient (API) from a medicinal product to the environment. The first is related to the process of drug metabolism in the human body. In this case, API may be excreted in both the metabolized and non-metabolized forms. The second and the more harmful way, is associated with the improper disposal of outdated and unused drugs through sanitation that is not dedicated to this kind of pollution [6,7]. A pharmacist's advice is an important and useful tool in improving the patient's awareness about the harmful impact of unused and/or outdated drugs on natural habitats. In some countries, medication labels contain detailed information regarding the special way to dispose of said medicinal products. Only 1.4% of patients returned unused drugs to their community pharmacy, according to an American study. Currently, the Food and Drug Administration (FDA) provides detailed recommendations for patients and health care professionals referring to the disposal of medications. Particularly of interest is the list of medications which should be flushed when possible. This category includes drugs which are particularly dangerous for adults, children, and pets, such as formulations containing fentanyl, morphine, oxycodone or hydrocodone, or those with high addictive potential [8].
In general, pharmaceutical care contributes to patient pharmacotherapy on an individual level. One must remember, while this relationship is individual, it encompasses the perspectives of the entire population and their impact on the environment. Due to this, advising patients about the management of unused and/or outdated medicinal products is an important service within the pharmaceutical care concept. Proper waste management in the community pharmacy setting should be considered as one of the pillars of pharmaceutical public health. Pharmaceutical care, both on an individual and a broad population level remains challenging, especially from a legal point of view [9].
The aim of this paper is to provide a brief review of the various managements of unused and/or outdated medicinal products in the Polish community pharmacy setting. The broad perspective of this paper aims to familiarize the multidimensional character of this topic, not only from a legal point of view but also from the perspective of an environmental hazard. Finally, we offer practical recommendations for pharmacists, thus our paper is not only one of scientific deliberations but might prove useful for both practitioners and governors.
Drug disposal – an international perspective
Drug policies can differ significantly in each country, even among the members of the EU. These differences can also be seen in the context of drug disposal. The Health Information and Quality Authority, an independent Irish consulting body, has noted that one of the most well-established ways to dispose of medications is to allow the patients to offer them back to the community pharmacy. The Irish Authority strongly recommends that in each phase of drug utilization, one remembers the vital importance of protecting public health and avoiding any contamination of the natural environment. Drug disposal remains a difficult and time-consuming process because of various legislative procedures required by pharmaceutical law. In Ireland, pharmacists must collect information about the date of disposal, the name, type and strength of the returned medication or in rare situations, the identity of the patient for whom medications were prescribed [10]. In the United Kingdom, the Royal Pharmaceutical Society focuses on how teamwork is the most useful tool to dispose of medications safely. Apart from general recommendations, they have released an official document which mandates the implementation of standard operational procedures (SOP) including a detailed description of each phase of drug disposal especially in the specific conditions of community pharmacy. Every SOP should be adjusted to staff availability and thus defines precisely the responsibilities of all team members. One should bear in mind that the disposal of controlled drugs, narcotics or certain psychotropic medications, are described in different sets of legislative acts and, are generally more complex than the utilization of non-controlled medications [11,12]. In summary, it should be emphasized that drug disposal is one of the obligations of a community pharmacy and safety utilization remains a significant part of the pharmacy profession as strongly recommended by the National Health Service in England [13]. Contrary to this, the disposal of drugs in the United States is much more complex due to the fact that the majority of regulations about drug disposal are described in the state law. This may lead to situations where the federal recommendations and the state law differ. Nevertheless, each state has underlined the importance of protecting the natural environment and emphasize that proper medication management remains the strongest way to reduce the amount of hazardous waste related to medication disposal [14].
Legal situation in Poland
Health care policies, and, consequently, drug policies, remain the matter of national policy. Only some aspects are regulated by the European law, such as those referring to regulatory affairs and law initiatives like the Falsified Medicines Directive (FMD). The regulations associated with drug utilization are found under the national law, particularly in the pharmaceutical law and acts approved by the Minister of Health [15].
According to this, from a legal point of view, owners and pharmacy managers have no legal duty to transfer unused and/or outdated medicinal products from patients to companies that specialize in waste utilization. Despite this, many community pharmacies offer the possibility to collect their patient's unused medicinal products. This is not a standard part of pharmaceutical care, but rather a marketing incentive aimed at attracting new clients and drawing attention to other services available in a particular community pharmacy. Patients can easily find a list of community pharmacies where they may return unused or outdated medicinal products by looking at the websites of regional pharmaceutical chambers or their local government. Rarely, public authorities organize the collection of unused medicinal products [16,17,18]. This additional service remains particularly important in rural areas where patients may have difficult access to a community pharmacy. Similarly, there can be problems when collecting mercury thermometers because these kind of tools should be stored under special conditions which prevent the non-intended release of mercury [19]. Outdated and unused drugs that are part of the stocks of a particular community pharmacy, should be disposed of properly by the manager. The pharmacy manager is responsible for waste management and should transfer these items to companies specialized in the disposal of medicinal products. According to current regulations, the manager should document this transfer, including all necessary information about the drug series, brand name and dosage of active pharmaceutical ingredient (API) [20]. The situation is more complex with regards to certain narcotics and psychotropic medications as the disposal of these drugs must be done with direct cooperation of the pharmaceutical inspectorate [21,22]. In each case, the pharmaceutical authorities should secure and mark the particular set of medicinal products before formal utilization.
In countries where pharmaceutical care is well-established and the role of the pharmacist is focused more on the clinical aspects rather than distribution, we can find more flexible regulations, e.g. standard procedures covering various aspects of how a pharmacist should deal with hazardous waste in the routine setting. This situation is drastically different when compared to current Polish pharmacy practice. This problem was widely discussed in the UK when the Royal Pharmaceutical Society introduced recommendations of which the main goal was to improve the safety in community pharmacies [23].
Unused and/or outdated drugs - the lack of detailed procedures in Poland
As mentioned before, Poland currently lacks detailed procedures referring to waste management that is approved by the official authorities or academic bodies with regards to pharmacies. The lack of these procedures is dangerous not only for pharmacists and pharmacy technicians, but also the supportive staff, which are often directly responsible for preparing medical waste for disposal. Farmacja.net, an internal portal that serves as one of the many platforms of professional communication between pharmacists in Poland; published a study where 6% of respondents declared that they had incurred needle stick injuries while emptying the waste container [24].
In accordance with international standards approved by the Royal Pharmaceutical Society, standards which are strictly related to British pharmaceutical practice, we suggest the authorial procedures which may prove useful for Polish pharmaceutical practice. We believe that introducing standards and set procedures will improve the safety of the staff of community pharmacies. While some aspects of recommendations mentioned below may also be used in the hospital pharmacy setting, we would like to emphasize that our suggestions are dedicated mostly to community pharmacy setting. These recommendations should be understood as a glimpse of the future of pharmaceutical practice in Poland.
Unused and/or outdated drugs – multi-phase protocol
The first phase of returning unused and/or outdated drugs to a community pharmacy should begin with a well-structured interview with the patient. Pharmacists may use questionnaires to collect detailed information about the collected waste, allowing them to quickly identify hazardous substances, assess risks to personnel, and providing the opportunity to protect the natural environment. A useful set of questions is suggested and summarized in Table 1.
Table 1. An example of a questionnaire completed by the patient with marked answers (Piotr Merks et al.).
|
Are you sure that you are returning only medications? |
YES/NO |
|
Are you sure that there are no needles, blades or other sharp objects in your |
YES/NO |
|
Are you sure that there are no substances which may endanger life or be harmful to health inside your medication bag? |
YES/NO |
After the patient fills out the questionnaire, and speaks with the pharmacist, a decision must be made whether the returned medications can be accepted by the community pharmacy or should be directed to a more appropriate institution. In Table 2 we suggest guidelines that can be applied in the community pharmacy setting by professional staff.
Table 2. The list of products that can be accepted by the pharmacy (Piotr Merks et al.).
|
Yes, we accept! |
We apologize, but we cannot accept! |
|
All outdated and unused drugs including the following: |
Needles or other sharp objects. |
|
Tablets |
Accessories for dialysis |
|
Liquid formulations |
Chemicals |
|
Ampules |
Veterinary agents |
|
Powders |
Pesticides |
|
Inhalers |
Paints, solvents and industrial oils |
|
Ointments |
All other products that are not drugs |
The place in the community pharmacy where unused and/or outdated drugs will be stored, should be situated out of reach, with no free patient access, otherwise, pharmaceutical staff will not have full control over the process of accepting medications. The patients should be aware that the container will be opened by the staff member only after a short interview and assurance that the product intended to be returned fulfills all inclusive criteria (e.g. Table 2). The containers should allow for complete sealing and safe transportation to the utilization company. It should be emptied regularly in order to reduce the risks associated with excessive overloading. The container should only be emptied by trained personnel from a specialized company that deals with waste disposal. This entire process can be easily described in several steps, quickly understood and realized, while ensuring the safety of community or hospital pharmaceutical staff. In the opinion of the Authors of this paper, these steps are necessary and their implementation in routine settings will facilitate waste management.
1. Prepare a place where you can safely check the items returned by the patient.
2. Make sure that the container can be opened and that it has sufficient free space available inside.
3. Ask the patient to carefully read the instructions and to respond to all questions included in the questionnaire.
4. If you must pick up the medication bag containing the unused and/or outdated drugs, make sure to grab it by both handles. Do not hold the bag directly in your hands. Place the bag in the space you have prepared early to examine its contents.
5. Check carefully that there are no needles, blades, or other sharp objects, as these items cannot be accepted by the community pharmacy. If you have problems classifying the objects, or deciding whether or not you can accept a particular item, you may contact relevant institutions like the Ministry of Environment or the company responsible for waste management. You should bear in mind the potential toxicity of certain substances, as some of the returned drugs may contain active pharmaceutical ingredients that are particularly harmful to human beings.
6. If the patient wants to return any narcotics or psychotropic medications, please remember about the special procedures associated with utilizing these kind of medications, described in detail in the pharmaceutical law.
7. Place the acceptable returned medicinal products into the disposal container.
8. Wash your hands.
9. All drugs returned to community or hospital pharmacies should be kept in a safe place away from unauthorized access.
10. Remember to regularly empty the container. In this way, you can avoid the accumulation of dangerous waste in the work area.
11. All returned drugs must be disposed of properly by institutions certified by the appropriate authorities.
12. The collection of outdated and unused medicinal products should be confirmed by a written protocol signed by the manager of the particular pharmacy and a representative of the specialized waste management company.
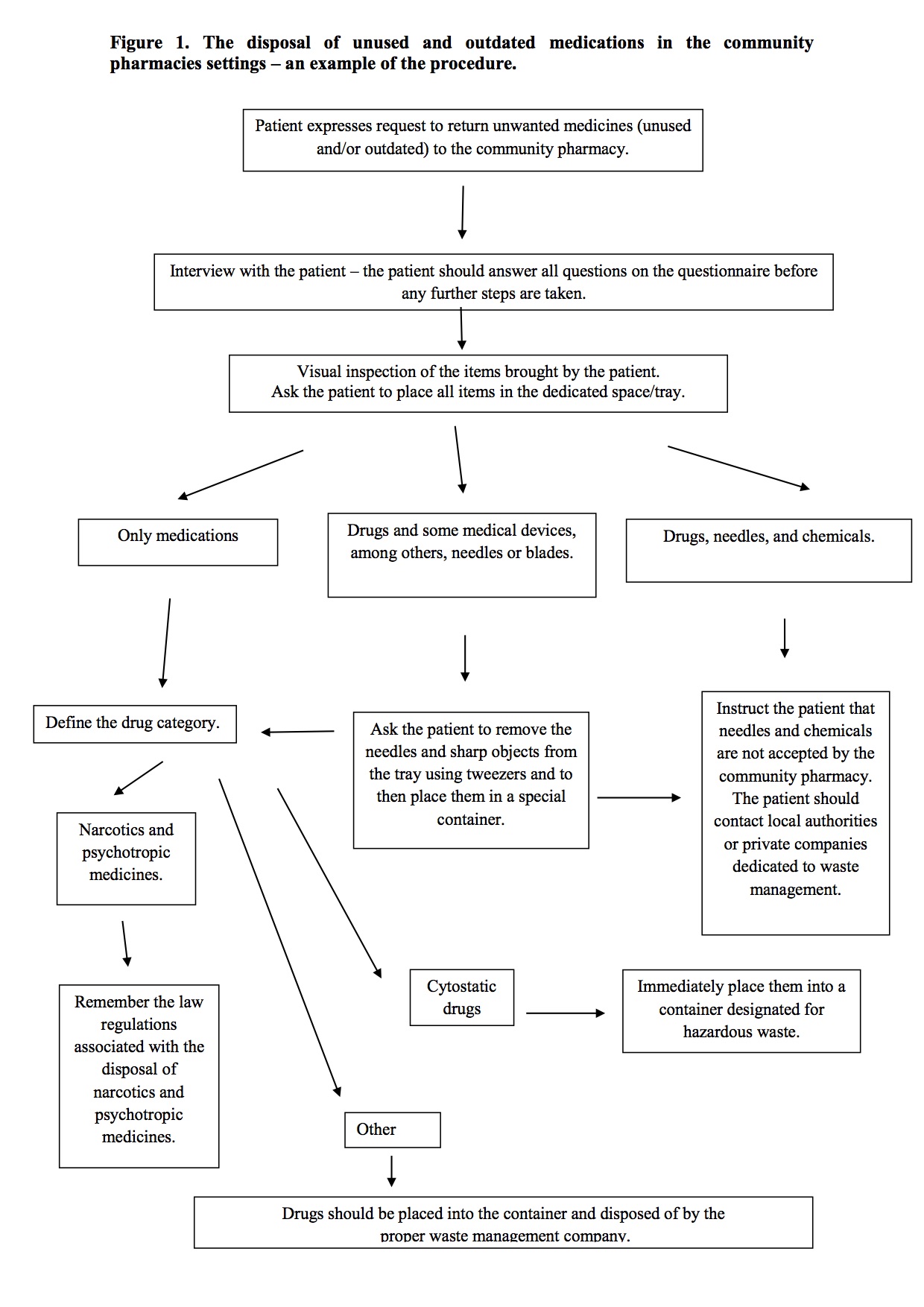
An example of the procedure is presented in Figure 1.
So far we have described standards and protocols as essential tools to ensure the safety of personnel working in the community pharmacy. It is essential to remember that while counseling patients on pharmacotherapy, pharmacists are also provided the opportunity to assess the patient's awareness of the possible harmful effects of drugs on the natural environment. Educating patients on how to reduce these risks, for example by returning unused medications, is vital. All members of pharmacy staff are responsible for waste management, thus, everyone must be trained on how to deal with outdated and unused drugs which have been returned by patients. All phases of accepting the drugs that have been returned by a patient must be conducted by the same employee. It is unacceptable for this process to be split and undertaken by more than one professional. Furthermore, this procedure, once introduced into the community pharmacy setting, must be regularly audited and the level of knowledge of the pharmacy staff should be carefully evaluated. Necessary revision courses should be systematically conducted to achieve not only a high level of theoretical knowledge but also improve the implementation of new standards in the routine pharmacy practice. All these items contribute to improving patient safety, safety of the pharmacy staff and better protection of our environment.
Conclusions
Undoubtedly, a more effective waste management, in the context of unused and/or outdated medicinal products, is essential, not only from the environmental point of view, but it also contributes to more cost-effective drug utilization in the healthcare system. Continuous education can improve patient awareness, and, consequently lead to more effective management of the patient's own pharmacotherapy. Patients should be encouraged to stop gathering excessive stocks of drugs, especially elderly individuals. In this context, the medicinal package is particularly useful, as the medical information found on the label remains accessible to the patients in every moment and serves as a helpful reminder of the information obtained during the consultation at the community pharmacy. A crucial step that is necessary to improve the quality of waste management in the Polish community pharmacy settings, remains to introduce new protocols and standards. Procedures should protect the natural habitat against toxic substances, but also improve the patients and pharmacy staff's level of safety. Pharmaceutical care is defined as sets of pharmaceutical services contributing to patient health, but should also be seen in a new role – as services which help us protect the environment. While this perspective on pharmaceutical care is not well-known nor well-established, it corresponds with the concept of pharmaceutical public health and the definition of the community pharmacy as a place particularly dedicated to promoting public health. Practical regulations about the implementation of new laws, research aimed at understanding the patient’s perspective on herein described issues, and the active promotion of proper attitudes is essential to the future of Polish pharmaceutical care.
[1]. MZ opublikowało nową listę leków zagrożonych brakiem dostępności - Farmacja. http://www.rynekzdrowia.pl/Farmacja/MZ-opublikowalo-nowa-liste-lekow-zagrozonych-brakiem-dostepnosci,165593,6.html. Accessed January 18, 2017.
[2]. Raport: polski rynek wyróżnia się dużo większą liczbą aptek - strona 2 - KOMENTARZE I OPINIE. http://www.rynekaptek.pl/wywiad/raport-polski-rynek-wyroznia-sie-duzo-wieksza-liczba-aptek,15852_1.html. Accessed January 18, 2017.
[3]. MZ: 4 mln opakowań bezpłatnych leków dla seniorów - Polityka zdrowotna. http://www.rynekzdrowia.pl/Polityka-zdrowotna/MZ-4-mln-opakowan-bezplatnych-lekow-dla-seniorow,167441,14.html. Accessed January 18, 2017.
[4]. Kuspis DA, Krenzelok EP. What happens to expired medications? A survey of community medication disposal. Vet Human Toxicol. 1996, 38:48–49.
[5]. Raifman J, Chetty T, Tanser F, et al. Preventing unintended pregnancy and HIV transmission: effects of the HIV treatment cascade on contraceptive use and choice in rural KwaZulu-Natal. J Acquir Immune Defic Syndr. 2014;67 Suppl 4(Suppl 4):S218-27. doi:10.1097/QAI.0000000000000373.
[6]. Lubick N. Drugs in the environment: do pharmaceutical take-back programs make a difference? Environ Health Perspect. 2010;118(5):A210-4. doi:10.1289/ehp.118-a210.
[7]. Boxall ABA. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116. doi:10.1038/sj.embor.7400307.
[8]. Disposal of Unused Medicines: What You Should Know. FDA. http://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/ensuringsafeuseofmedicine/safedisposalofmedicines/ucm186187.htm. Accessed January 18, 2017.
[9]. Green Pharmacy Practice. Report for pharmacists. FIP. https://www.fip.org/bangkok2014/files/static/Draft_document_GREEN_PHARMACY_PRACTICE.pdf. Accessed January 18, 2017.
[10]. Health Information and Quality Authority. Medicines Management Guidance. https://www.hiqa.ie/system/files/Medicines-Management-Guidance.pdf. Accessed 28 January 2017.
[11]. The Safe and Secure Handling of Medicines: a team approach. Royal Pharmaceutical Society of Great Britain. http://www.rpharms.com/support-pdfs/safsechandmeds.pdf. Accessed 28 January 2017.
[12]. New guidance on safe practice in the disposal of Controlled Drugs. The Pharmaceutical Journal. http://www.pharmaceutical-journal.com/career/career-feature/new-guidance-on-safe-practice-in-the-disposal-of-controlled-drugs/10007150.article. Accessed 28 January 2017.
[13]. Disposal of unwanted medicines. https://psnc.org.uk/services-commissioning/essential-services/disposal-of-unwanted-medicines/. Accessed 28 January 2017.
[14]. Pharmaceutical waste management: Compliance with environmental regulations. Modern Medicine Network. http://drugtopics.modernmedicine.com/drug-topics/news/modernmedicine/modern-medicine-feature-articles/pharmaceutical-waste-management-com. Accessed 28 January 2017.
[15]. Merks P, Swieczkowski D, Byliniak M, et al. The European Falsified Medicines Directive in Poland: background, implementation and potential recommendations for pharmacists. Eur J Hosp Pharm. doi:10.1136/ejhpharm-2016-000970 (ahead of print).
[16]. Zbiórka przeterminowanych leków - Oddaj odpady : EkoCentrum - Czysty Kraków Lepsze Życie. http://www.ekocentrum.krakow.pl/1,zbiorka-przeterminowanych-lekow.html. Accessed January 18, 2017.
[17]. Zbędne, przeterminowane leki tylko do apteki! https://www.doz.pl/czytelnia/a2094-Zbedne_przeterminowane_leki_tylko_do_apteki. Accessed January 18, 2017.
[18]. Nie wyrzucaj. Przynieś do apteki - przeterminowane leki [LISTA APTEK]. http://bialystok.wyborcza.pl/bialystok/1,35241,18860988,nie-wyrzucaj-przynies-do-apteki-przeterminowane-leki-lista.html Accessed January 18, 2017.
[19]. Punkty zbiórki przeterminowanych leków i termometrów rtęciowych - Stolica czystości. https://czysta.um.warszawa.pl/punkty-zbiorki-przeterminowanych-lekow-i-termometrow-rteciowych. Accessed January 18, 2017.
[20]. Przeterminowane leki apteka przekazuje do utylizacji - Styl życia - Gazeta Prawna - wiadomości, podatki, prawo, biznes i finanse. http://serwisy.gazetaprawna.pl/zdrowie/artykuly/573281,przeterminowane-leki-apteka-przekazuje-do-utylizacji.html. Accessed January 18, 2017.
[21]. Utylizacja leków odurzających i psychotropowych - PRAWO. http://www.rynekaptek.pl/prawo/utylizacja-lekow-odurzajacych-i-psychotropowych,2720.html. Accessed January 18, 2017.
[22]. Rozporządzenie Ministra Zdrowia z dnia 27 lutego 2012 r. w sprawie szczegółowych warunków i trybu postępowania ze środkami odurzającymi, substancjami psychotropowymi i prekursorami kategorii 1, ich mieszaninami oraz produktami leczniczymi, zepsutymi, sfałszowanymi lub którym upłynął termin ważności, zawierającymi w swoim składzie środki odurzające, substancje psychotropowe lub prekursory kategorii 1. http://www2.mz.gov.pl/wwwmz/index?mr=m0&ms=&ml=pl&mi=904&mx=0&mt=&my=9&ma=019400 Accessed January 18, 2017.
[23]. The Handling of Medicines in Social Care. Royal Pharmaceutical Society. https://www.rpharms.com/social-care-settings-pdfs/the-handling-of-medicines-in-social-care.pdf. Accessed January 18, 2017.
[24]. Społeczność farmaceutów i techników farmaceutycznych. http://farmacja.net/home. Accessed January 18, 2017.