Severe allergic asthma treatment in Poland
-
Copyright
© 2016 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Severe asthma is a heterogeneous disease, which, as it is now believed, requires a personalized therapy dependent on phenotype. Omalizumab, a humanized monoclonal antibody that specifically binds to free immunoglobulin E, proved to be particularly effective in the treatment of allergic asthma. Because this treatment is very expensive, in Poland it is reimbursed under the drug program: “Omalizumab treatment of severe, allergic, IgE-dependent asthma”. One of the main features of drug programs is precise defining the group of patients, which may be subject to treatment. The current experience shows that it significantly increase the efficacy of the therapy. On the other hand, however, may adversely affect the possibility of treating patients with difficult access to specialized centres.
Introduction
Asthma is a common disease for many years treated as a homogenous pathology. The only differentiation was between intrinsic asthma, where no triggers were known, and extrinsic, were reaction to allergens was thought to be responsible for airway inflammation related symptoms. With the emergence of new data on the pathogenesis of asthma and observations on the diversity of the clinical picture of this pathology, asthma started to be perceived as a heterogeneous disorder, including discrete units with specific clinical course, physiology and pathogenesis, requiring personalized therapy. To undersign this heterogeneity Wenzel in 2012 [1] introduced the term “severe asthma syndrome” and described different asthma phenotypes. One of them was allergic asthma. This is the subgroup representing around 40% of all severe asthmatics. And although it is already known that even this subgroup is not homogeneous, most patients are characterised by raised IgE positive cells number with increased levels of specific and total Immunoglobulin E (IgE). IgE plays a central role in the pathogenesis of allergic diseases, including asthma. For this reason, IgE-mediated immunologic pathways represent an attractive target for therapeutic agents in asthma. Omalizumab is a recombinant humanized immunoglobulin G1 monoclonal antibody that binds IgE with high affinity. The efficacy and safety of omalizumab in moderate-to-severe persistent allergic asthma were confirmed in numerous multicenter clinical trials [2,3]. This is why it is approved for use as an add-on treatment in patients six years of age and older with severe persistent asthma where allergic sensitization was demonstrated by positive skin testing or in vitro testing for allergen-specific IgE to a perennial allergen and asthma symptoms are inadequately controlled with inhaled glucocorticoids (ICS), and with the documented history of frequent severe asthma exacerbations despite high doses of ICS in combination with long-acting Beta2-agonists (LABA). In adolescents and adults treatment is recommended in patients with reduced lung function (FEV1<80%).
Since March 2013 omalizumab therapy became available in Poland as part of the drug program.
Drug program: definition and management
Innovative therapies (primarily biological therapies) in Poland are reimbursed within drug programs [4]. According to definition: “the drug program is guaranteed compensation, including therapies with innovative, expensive active substances which are not financed by other guaranteed benefits. The treatment is carried out in selected disease entities and includes strictly defined group of patients”. Omalizumab treatment in Poland is held within the program: “Omalizumab treatment of severe, allergic, IgE-dependent asthma”. The drug program inclusion and exclusion criteria, as well as contraindications to the use of omalizumab, a list of tests to qualify a patient for treatment, and then to subsequent administrations of the drug, the scheme of monitoring visits and omalizumab dosing, as well as specification of treatment duration can be found in Annex B.44 to the Notice of the Minister of Health of 28 October 2015 [5]. The inclusion and exclusion criteria of this program are listed in table 1.
Table 1. Inclusion and exclusion criteria in omalizumab drug program
|
Inclusion Criteria |
|
|
Adult and adolescent patients with severe, uncontrolled allergic asthma (per GINA guidelines), allergy to perennial allergens confirmed by positive results of skin prick or specific IgE tests |
|
The need to use high doses of ICS (>1000 mcg of beclomethasone dipropionate (CFC) per day or equivalent) in combination with a second controller (i.e. LABA, LTRA, theophylline) |
|
Frequent use of OCS in the past, including the last 6 months |
|
Total serum IgE levels 30-1500 IU / ml |
|
Unequivocal in vitro reactivity to a perennial allergen in patients with total serum IgE (tIgE) levels below 76 IU/ml |
|
Meeting of at least three of the following criteria: a) Uncontrolled asthma symptoms (lack of asthma control with ACQ > 1,5 points) b) Three or more episodes of exacerbations a year requiring systemic corticosteroids or increase their current dose. c) Hospitalization in the past 12 months due to asthma exacerbation d) Near fatal asthma episode in the past e) Persistent airflow limitation (forced expiratory flow in one second (FEV1) < 60% predicted of normal or daily variability of peak expiratory flow (PEF) > 30%) |
|
Weight 20-150 kg |
|
Non-smoker |
|
The exclusion of other than allergic reactions to inhaled perennial allergens reasons causing severe asthma |
|
Exclusion criteria |
|
|
Exacerbations during treatment with omalizumab if their number is equal to or greater than in the period prior treatment year follow |
|
Criteria of efficacy not met: a) Response to therapy in GETE less than excellent (complete control of asthma)/good (marked improvement) b) Meeting 2 of the following 3 criteria:
|
|
Current smoker |
|
Noncompliance or poor adherence |
|
Initiation of therapy with immunosuppressive drugs, anticancer, infusions of immunoglobulins or other biological agents |
|
The occurrence of any contraindications to the use of omalizumab |
|
Pregnant or breastfeeding woman |
Omalizumab treatment must be discontinued if the patient meets any of the exclusion criteria. Otherwise, the treatment should not be stopped until after 24 months. In this case, the patient should be monitored for a minimum of six months. In the event of relapse treatment can be applied again.
Candidate patients for the omalizumab drug program treatment have to perform the following examinations and tests: total IgE (where total IgE is < 76IU/ml the level of specific IgE must be also determined), vital sings and physical examination (including weight), lung function test, and safety laboratory (morphology, CRP, OB, ALT, AST, creatinine, urea). Pregnancy test must be performed in all women of childbearing potential. Asthma control Questionnaire (ACQ) and Asthma Quality of Life Questionnaire (mini AQLQ) has to be completed as a part of the preliminary evaluation. The result of skin prick tests may be historic. During this visit, an overall assessment of the patient`s health is carried out with the analysis of all asthma medicines taken, as well as the number and seriousness of asthma exacerbations in the previous year.
Questionnaires are completed then on every visit associated with omalizumab administration. Similarly to lung function testing and patient overall assessment.
The key points to assess patients are monitoring visits after 16weeks and then every 52 weeks since the start of treatment, when making an evaluation of the effectiveness and safety of the therapy. Only patients meeting all of the mentioned below efficacy criteria (responders) can continue treatment:
- the number of exacerbations less than before treatment,
- Global Evaluation of Treatment Effectiveness (GETE): good or excellent asthma control,
- improvement of quality of life (an increase of greater than 0,5 points in the miniAQLQ score),
- improvement of asthma control (decrease of greater than 0,5 points in the ACQ score),
- oral corticosteroids (OCS) dose reduction not less than 5 mg per day (only patients treated with OCS before enrolment).
Drug program: therapy centres and treated patients
On 26 October 2012 announcement of the Minister of Health was published on the introduction of drug program for omalizumab treatment of severe, allergic, IgE-dependent asthma. Treatment was started in March 2013 – the first patient was qualified for therapy 19.03.2013. About 461 patients are currently treated with omalizumab (data from the beginning of March 2017). So far treatment was completed in 99 patients.
Since July 2016, the National Health Fund also granted the option to suspend treatment. It applies to patients treated with omalizumab for at least 2 years. “Suspension” means that patients who discontinued omalizumab can be re-treated in the event of worsening asthma control without the need for re-qualification. Now 14 patients are being suspended.
Currently treatment with omalizumab is carried out by the 42 treatment centers (health care providers) who have concluded for this purpose additional contract with the National Health Fund. It seems that access to treatment should be good, because there is it least one center located in each of 16 regions (Fig.1).
Fig. 1. Centers (health care providers) implementing the drug program: “Omalizumab treatment of severe, allergic, IgE-dependent asthma”
Unfortunately, it looks different, when the implementation of the program in individual regions is assessed (tab. 2; fig.2). Because there are clear disproportions between the regions with the largest number of patients in: Łódzkie, Wielkopolskie, Mazowieckie, Małopolskie, Śląskie and Lubuskie (more than 40 patients treated) and the lowest (6-9 patients treated) in: Świętokrzyskie, Podlaskie, Podkarpackie and Opolskie.
Tab. 2. The current number of patients in the drug program: “Omalizumab treatment of severe, allergic, IgE-dependent asthma”
|
Region |
No of patients treated |
No of patients suspended |
No of patients completed |
No of rejected applications |
|
1. Dolnośląskie |
30 |
0 |
6 |
4 |
|
2. Kujawsko-pomorskie |
16 |
0 |
3 |
3 |
|
3. Lubelskie |
23 |
0 |
10 |
4 |
|
4. Lubuskie |
44 |
0 |
4 |
11 |
|
5. Łódzkie |
99 |
1 |
8 |
22 |
|
6. Małopolskie |
46 |
1 |
13 |
9 |
|
7. Mazowieckie |
50 |
10 |
10 |
10 |
|
8. Opolskie |
9 |
0 |
1 |
4 |
|
9. Podkarpackie |
8 |
0 |
10 |
1 |
|
10. Podlaskie |
8 |
0 |
9 |
11 |
|
11. Pomorskie |
15 |
0 |
0 |
3 |
|
12. Śląskie |
44 |
0 |
4 |
11 |
|
13. Świętokrzyskie |
6 |
0 |
9 |
4 |
|
14. Warmińsko-mazurskie |
22 |
1 |
3 |
1 |
|
15. Wielkopolskie |
53 |
1 |
5 |
7 |
|
16. Zachodniopomorskie |
18 |
0 |
6 |
6 |
|
TOTAL |
491 |
14 |
101 |
111 |
Fig.2 The current number of patients in the drug program: “Omalizumab treatment of severe, allergic, IgE-dependent asthma” - broken down by region.
If the number of treated patients will refer to the total population (ratio: No of treated/total population/1 mln) disproportions are even more evident, although the distribution of patients is somewhat different, with the largest number treated in Lubuskie. Pareto chart also allows to show that 40% of patients are treated in just two regions: Lubuskie and Łódzkie.
And even if the number of treated patients will refer to the total population, it does not change this obvious disproportion (Fig.3)
Fig. 3 Pareto chart showing the ratio: No of treated/total population/1 mln in particular regions.
In 2010 a group of experts preparing the program of omalizumab treatment in severe allergic asthma, based on data from other countries, but also the results of research: “Epidemiology of allergic diseases in Poland” as well as the number of patients with severe allergic asthma with serum IgE in the range of 30-1500, estimated the number of patients eligible for treatment with omalizumab at about 1,000 [6]. This estimate, along with the above presented a very uneven distribution of patients treated with omalizumab in different provinces may indicate that not all patients requiring treatment are qualified for it. Efforts should be made to change this situation.
The efficacy and safety of omalizumab treatment
Preliminary studies carried out on a group of 85 patients (data not published) who first completed the first-year course of treatment confirmed its high efficiency: excellent and very good response to therapy after 16 and 52 weeks has been observed in 95,1% and 93,9% of patients respectively. Asthma exacerbation rate was reduced by 66%. Also, scores on quality of life questionnaires (AQLQ) and asthma control (ACQ) has improved. Omalizumab treatment can also be regarded as safe with reported adverse effects being relatively rare. However there were some cases in which adverse events led to discontinuation of treatment – mainly from the musculoskeletal system (joint and muscle pain, joint swelling). Other side effects include headache, dizziness, leukopenia, hair loss, lichen planus, recurrent furunculosis or feeling of tiredness. There were, however, no cases of anaphylaxis after omalizumab administration.
The compatibility of the omalizumab drug program with the current guidelines of severe asthma treatment (GINA) and Summary of Product Characteristics (SPC)
Considering issues relating to the omalizumab treatment of severe asthma in Poland one would be wondering how the treatment is in accordance with the current guidelines. According to GINA 2017 [7] add-on anti-immunoglobulin E (omalizumab) treatment should be started in: “patients aged ≥ 6 years with moderate or severe allergic asthma that is uncontrolled on Step 4 treatment “
In omalizumab drug program in Poland only children from 12 years of life are treated. They constitute about 5% of all treated with omalizumab (about 23 patients). In the expert opinion the group of children aged 6-11 years old requiring administration of anti IgE would be even less numerous. This is due to some distinctiveness in childhood asthma, as well as the diagnostic problems. Because the product characteristics allows the use of omalizumab in children aged 6 years of age, treatment can be carried out and settled within homogeneous groups of patients (JGP – jednorodne grupy pacjentów).
Eligibility criteria for the treatment in the drug program, as in GINA document, is severe allergic asthma, uncontrolled on Step 4 treatment (the need to use high doses of ICS in combination with a second controller ). The difference is, that in drug program a necessary criterion for inclusion is frequent use of OCS in the past, including the last 6 months. It should however be stressed, that chronic OCS treatment is not inclusion criteria. So that in practice every patient with severe exacerbations (and it is one of the characteristics of severe allergic asthma), may be treated with omalizumab. One can also note, that according to the SPC omalizumab should be used in patients with “the documented history of frequent severe asthma exacerbations despite high doses of ICS in combination with long-acting Beta2-agonists (LABA). According to the SPC omalizumab should only be used if lung function is reduced ((FEV1<80%). This very restrictive record is not found in the description of the drug program. This allows the use of omalizumab treatment in patients which are characterized by reversible airway constriction.
Drug program expenses incurred by the National Health Fund
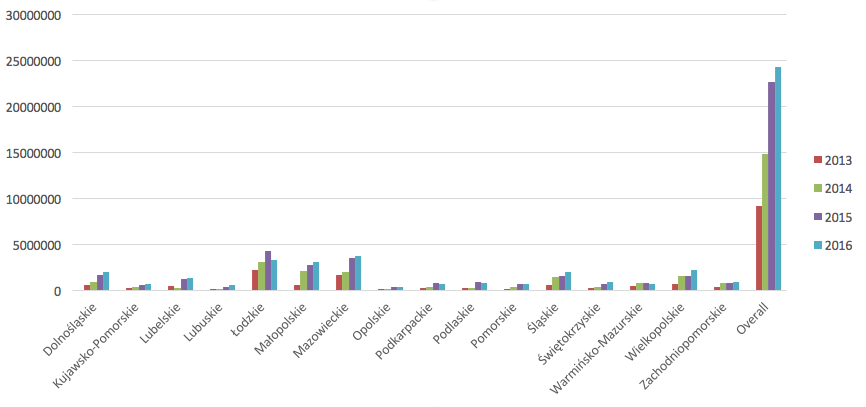
In Poland, as of 31.06.2016, there were 85000 patients included and treated in 75 various drug programmes. The overall costs of those programmes in 2016 was 2 850 211 166 PLN. Costs of therapy within the omalizumab programme in different voivodeships is presented in the figure 4.
Figure 4. Costs of therapy within the “Omalizumab treatment of severe, allergic, IgE-dependent asthma” drug programme in years 2013-2016, in PLN.
Conclusions
The treatment of severe asthma with omalizumab in Poland is reimbursed within the drug program, which uses certain items of clinical trials, among others, precisely defines the inclusion and exclusion criteria. Apparent advantages of such qualifications for the treatment is high efficiency of the therapy most likely resulting from a more thorough initial evaluation of patients. Monitoring visits allow in turn for more detailed assessment of the efficacy, safety, and cost of the therapy. Disadvantages are: more difficult access to treatment (which is carried out only in highly specialized centers), and, fairy rigid description of the drug program – it does not change despite upgrades both in the SPC and guidelines (GNA). This is what for instance makes this treatment excluded for children aged 6-11 years.
It should be emphasized that the introduction of severe asthma treatment program in Poland allowed to treat our patients in accordance with the current standards.
- Wenzel S.: Severe asthma: from characteristics to phenotypes to endotypes. Clin. Exp. Allergy. 2012;42:650-658
- Holgate S.T., Chuchalin A.G., Hébert J., Lötvall J., Persson G.B., Chung K.F. et al.: Omalizumab 011 International Study Group. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin. Exp. Allergy 2004; 34(4):632–638.
- Humbert M., Beasley R., Ayres J., Slavin R., Hébert J., Bousquet J. et al.: Benefits of omalizumab as add on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60(3):309–316
- NOTICE OF THE MINISTER OF HEALTH of 26 October 2012 on the list of reimbursed drugs, foodstuffs intended for particular nutritional uses and medical devices at the date of 1 November 2012. Access: http://www.mz.gov.pl/leki/refundacja/lista-lekow-refundowanych-obwieszczenia-ministra-zdrowia/obwieszczenie-ministra-zdrowia-z-dnia-26-pazdziernika-2012-r
- Annex B.44. to the Notice of the Minister of Health of 28 October 2015 on the list of reimbursed drugs, foodstuffs intended for particular nutritional uses and medical devices at the date of 1 November 2015)
- Bodzenta-Lukaszyk A., Chazan R., Fal A., Kruszewski J., Kuna P., Kupryś-lipińska I. et al.: Stanowisko Grupy Ekspertów Polskiego Towarzystwa Alergologicznego w sprawie programu terapeutycznego leczenia omalizumabem ciężkiej astmy alergicznej. Alergia Astma Immunol. 2010.15(3):132-133
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available from: www.ginasthma.org