Major Cardiovascular Consequences of Familial Hypercholesterolemia in Poland from the Economic Perspective
-
Copyright
© 2018 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Background. Familial hypercholesterolemia (FH) is a genetic disease characterised by high LDL-C level with other lipid fractions levels typically correct. The disease involves the accelerated development of arteriosclerosis and vascular complications, mainly coronary disease. Patients with FH have an increased risk of the acute coronary syndrome (ACS), ischemic stroke (IS) and peripheral artery disease (PAD).
Aims. This publication aims to discuss epidemiology and costs associated with three major vascular complications of FH: ACS (including myocardial infarction, MI), IS and PAD. In case of lack of specific data for FH subpopulation, more general data will be discussed.
Results. Meta-analysis of six Polish population studies indicated that heterozygous FH morbidity in Poland is approximately 404/100,000 people what corresponds with approximately 150,000 patients. Each year 123 thousand ACS are reported in Poland of what approximately 62% is a MI. In European population FH is diagnosed in approximately 8.3% patients with ACS (in Polish subpopulation – approximately 11.4%). Total hospitalization costs due to ACS among patients with FH in 2016 equaled PLN 108-148 million, of what hospitalization cost due to MI was approximately PLN 67-91 million. Indirect medical costs during first 36 months after MI in patients with FH may be estimated for PLN 128-175 million and indirect costs of MI in patients with FH for PLN 257-684 million.
Conclusions. Early screening for familial hypercholesterolemia and efficient hypolipemic treatment are a chance for significant reduction of incidence of discussed vascular complications and their costs within the healthcare system. We were not able to identify any specific cost data for FH subpopulations of IS and PAD patients.
Familial Hypercholesterolemia
Familial Hypercholesterolemia (FH) is genetic disease inherited as an autosomal dominant feature and is characterised by high LDL-C level with other lipid fractions levels typically correct. The disease involves the accelerated development of arteriosclerosis and vascular complications, mainly coronary disease. Patients with FH have increased the risk of the occurrence of the acute coronary syndrome, ischemic stroke and peripheral artery disease.[1],[2] In majority of cases familial hypercholesterolemia is caused by LDL receptor gene mutation (LDLR, in approximately 79% patients with FH) less often by apolipoprotein B mutation (ApoB, approximately 5% of patients), proprotein convertase subtilisin kexin 9 (PCSK9, <1% of patients) and very rarely – mutations in low-density lipoprotein receptor adaptor protein 1 mutation (LDLRAP1). Remaining 15% of familial hypercholesterolemia cases are of multigenic or monogenic origin (among others APOE, APOB, SREBP2, STAP1 gene mutations). However, the mutations occurrence frequency was not yet stated.[3],[4]
Due to the inheritance model, two types of disease are distinguished: heterozygous (heFH) and homozygous familial hypercholesterolemia (hoFH). In hoFH very high total cholesterol level (>500 mg/dl in untreated patients) and arteriosclerosis development in early childhood are present and usually lead to death before 30 years of age. According to estimations frequency of hoFH worldwide is very small and equals from 1/160 thousand to 1/300 thousand people.[5]In heFH arteriosclerosis appears in men before 55 years old and in women before 60. Current ESC/EAS 2016 guidelines on dyslipidaemia management do not define an LDL-C value which defines hypercholesterolemia but distinguish five ranges of levels which state management model, i.e. lifestyle change or pharmacological treatment, depending on cardio-vascular risk category (small, moderate, high and very high). Level ranges include LDL-C:
Familial hypercholesterolemia epidemiology
Data on heFH morbidity in Europe and worldwide are limited due to lack of national registries and screening tests systems in most of the countries.[6],[7],[8] What is more, there are no uniform diagnostic criteria for hypercholesterolemia, and diagnosis of heFH may among others be based on Dutch Lipid Clinic Network criteria (DLCN; most common), Simon Broome Registry criteria, WHO criteria or genetic tests for mutations in LDLR, ApoB and PCSK9 genes.[5] Mentioned scales for heFH diagnosis are based on clinical and family interview, physical examination for characteristic symptoms of FH (among others tendinous xanthoma), LDL-C blood level and genetic tests for LDLR.
Until recently it was believed that heterozygous familial hypercholesterolemia concerns 1/500 people in Europe however current studies indicate a higher incidence of this disease.[9] In the systematic review which covered years 1990-2017 twenty one studies on familial hypercholesterolemia cases in the world were identified. Majority of the studies come from Europe, then from North America, Asia, Australia and Africa.7 The conducted meta-analysis of data concerning 2,458,456 people from 28 countries demonstrated that familial hypercholesterolemia occurs in 0.40% of worldwide population (95% CI: 0.29%; 0.52%) while FH frequency in the studies ranges from 0.05% to 5.62%. FH occurrence was diverse between age groups and region of origin. It was observed that frequency of FH occurrence increases with age. Such trend may be due to lack of screening tests for FH among children and adolescents and low detectability FH in the majority of countries. What is more LDL-C blood level increases with age what boosts the chance to diagnose FH with the use of most common DLCN criteria or Simon Broome Registry.[7] Detectability of familial hypercholesterolemia is highly diverse. Nordestgaard et al. (2013) estimated detectability of FH in 22 countries with the assumed morbidity at the level of 1/500 people. The highest detectability of familial hypercholesterolemia was reported in the Netherlands (71%) and Norway (43%), and then on Island (19%), in Switzerland (13%) and in Great Britain (12%). In other countries, this rate was lower than 10%, and in some countries, underdiagnosis of hypercholesterolemia was more than 99%. High detectability of FH in the Netherlands is an effect of efficient national screening program introduced in 1994 although in the context of new epidemiological data (morbidity 1/250 people) it may be assumed that FH detectability in the Netherlands is probably significantly lower than estimated.[6],[8]
In Poland occurrence of FH was assessed using epidemiological studies on cardiovascular diseases. The most recent data on FH morbidity come from a meta-analysis of data from 6 Polish population studies (POL-MONICA Cracow[10],[11], POL-MONICA Warsaw[10],[11], WOBASZ[12],[13], HAPIEE pilot[14], HAPIEE[14] and NATPOL 2011[15]) concerning a total of 39,768 people aged 20-74 diagnosed with FH based on DLCN criteria (Table 1).[16] According to published meta-analysis FH morbidity in Poland equals 404/100 thousand people (95% CI: 277-531/100 thousand people), what corresponds with approximately 1/250 people. Based on that number of patients with FH in Poland may be estimated for 150 thousand people. No data on detectability of familial hypercholesterolemia in Poland exists. However large epidemiological studies reported that in Poland undiagnosed cases of familial hypercholesterolemia constitute from 52% to 76%.[17],[18]
Table 1. Familial hypercholesterolemia morbidity in Poland according to DLCN criteria (definite and probable FH) in meta-analysis of Pajak et al. (2016)[16]
|
Trial |
FH morbidity per 100 thousand people (95% CI) |
|
POL-MONICA Cracow[10],[11] |
446 (264; 628) |
|
POL-MONICA Warsaw[10],[11] |
501 (313; 690) |
|
WOBASZ[12],[13] |
246 (163; 328) |
|
Pilot HAPIEE[14] |
538 (221; 856) |
|
HAPIEE[14] |
548 (396; 699) |
|
NATPOL 2011[15] |
231 (029; 434) |
|
Meta-analysis results |
404 (277; 531) |
Based on genetic, observational and intervention studies it was proven that hypercholesterolemia plays an important role in development of cardiovascular diseases. Available data from scientific studies indicate a direct association between LDL-C level and occurrence of acute coronary syndromes (ACS, including myocardial infarction, MI). Stroke and death due to cardiovascular causes.[19] It was demonstrated that familial hypercholesterolemia was associated with up to 20-fold higher risk of cardiovascular events due to long-term exposure of patients for the increased LDL-C level.[6],[20],[21] In patients with FH vs general population higher risk of the acute coronary syndrome, ischemic stroke or peripheral artery disease was reported.[22],[23],[24],[25]
Cardiovascular diseases constitute a significant economic burden for healthcare system and societies. In 2015 costs incurred for healthcare-associated with CVD in the European Union were approximately EUR 111 billion what is 8% of total expenditure for healthcare in EU. Expenditure associated with CVD among EU countries differ significantly – from EUR 48 per inhabitant in Bulgaria to EUR 365 per inhabitant in Finland. In Poland, in 2015 these expenditures were approximately EUR 115 per inhabitant and constituted 16% of total expenditure for healthcare.[26] Consideration of costs incurred by healthcare system alone leads to underestimation of real CVD cost. Socio-economic burden also includes costs of resources lost due to the disease and its consequences, i.e. costs of lost productivity of patients and their informal caregivers. In 2015 lost productivity costs due to CVD in UE were EUR 54 billion whereas informal care costs were EUR 45 billion. In Poland, these costs were estimated for EUR 2.5 billion and EUR 1.8 billion, respectively.[20]
The aim of this article was an attempt to estimate incidence and costs of major cardiovascular complications of familial hypercholesterolemia in Poland. As only in relation to ACS and MI data enabling estimating costs of these complications in a subpopulation of patients with FH is present, in terms of ischemic strokes and peripheral artery disease we would present total costs without distinguished FH subpopulation.
Acute coronary syndromes (ACS)
ACS risk increase in patients with FH vs general population
Premature coronary disease in patients with FH occurs in 78% of men and 73% of women while in patients without FH premature coronary disease is diagnosed in 33% of men and 37% of women.[27] At death, ACS was identified in 93% of patients with FH.[28] It is assessed that mutation associated with FH causes a 3.8-fold increase in the risk of coronary syndrome.[29] Cardiovascular events in patients with FH occur 10 years earlier than in patients without FH.[30] First MI was identified in patients with probable/ definite FH according to DLCN 14.6 years earlier than in patients without FH.[31] A chance of premature MI was 20.4-fold higher among patients with probable/ definite FH according to DLCN. Thereby occurrence of FH in a group with premature MI is 4-14-fold higher in comparison with general population.[31] Also, a risk of consecutive cardiovascular events in patients with FH is higher in comparison with patients without FH.[30],[31] Risk of MI in patients after ACS was 3.53-fold higher in patients with probable/ definite FH according to DLCN in comparison with patients without FH.[30] In Denmark in a group of patients after MI a risk of a recurrent event was 1.75-fold higher in patients with probable/ definite FH according to DLCN in comparison with patients without FH.[31] Similarly in Greece almost 2-fold increase of cardiovascular event risk after STEMI was observed in patients with probable/ definite FV according to DLCN in comparison with patients without FH.[32] In Norway mean length of life of patients with FH was 60 years, 69% experienced MI and 50% patients died due to cardiovascular causes.[28] In comparison with the general population standardized risk of death due to cardiovascular causes among patients with FH under 70 years of age was 2 times higher for men and 3 times higher for women.[33]
Acute coronary syndromes – epidemiology in Poland
In Poland, in 2013 219.[1] thousand people were diagnosed with coronary artery disease. Estimated incidence was 569.2 per 100 thousand inhabitants.[34] According to the prognosis of the Ministry of Health annual number of diagnosed cases is supposed to increase to 258.1 thousand in 2025.[34] In SILesian CARDiovascular database (SILCARD) which covers 4.6 million inhabitants, stable coronary disease (18.5%) was second after heart failure (20%) and before acute coronary syndrome (17.9%) cause of hospitalization due to cardiovascular causes in years 2006-2014.[35]
In Poland there is a register of acute coronary syndromes (PL-ACS), however, published aggregated data for ACS are relatively old, and the register includes a limited number of sites in Poland.[36] According to data from the national health Fund in 2013 123 thousand ACS were registered of which 62% was myocardial infarction. Estimated number of patients admitted for the first time ACS in 2013 was 98,509 patients of which 64% experienced a myocardial infarction. Death rate after a year from first ACS among patients admitted for the first time in 2013 was 13%.[36]
According to data from the National Database of myocardial infarction AMI-PL (Narodowa Baza Danych Zawałów Serca AMI-PL) in years 2009-2012 the number of hospitalized patients in Poland due to acute myocardial infarction ranged between 77.2 thousand in 2009 to 79.4 thousand in 2012. According to data from NIZP-PZH (National Institute of Public Health – National Institute of Hygiene) in Poland in 2011 approximately 87.5 thousand people experienced MI. A number of deaths in this year due to MI was 16.2, including 10.1 thousand (62.3%) deaths in hospital.[37] For 2010 standardized death rate due to MI was 36.7/100 thousand inhabitants (57.9/100 thousand in the male population and 20.7/100 thousand in female population).[38]
Men are more often hospitalized due to MI than women. In 2010 standardized hospitalization rates for both groups were respectively 260.3 and 103.5 per 100 thousand.[38] Risk of MI increases with age. In 2009 the highest contribution among hospitalized due to MI constituted people aged 50-79.[39] Median of age in men hospitalized due to MI in 2012 was 63 years and in women was 73 years.[37]
Risk of another hospitalization due to cardiovascular reasons among patients hospitalized in 2009 due to MI was 40% within a year from MI and 58% within 3 years. The most common cause of rehospitalization within a year from MI was stable coronary disease - 27%.[37]
ACS cost in Poland
Direct costs of treatment of cardiovascular disease were assessed in years 2009, 2011, 2012 in reports of ECDS[40], KPMG[39] and Instytut Ochrony Zdrowia (IOZ).[41]In 2009 according to ECDS report total direct cost was estimated for PLN 18.5 billions. KPMG report from 2011 indicated an estimation of the direct cost of cardiovascular diseases in Poland for PLN 15.3 billions. Cost of treatment of cardiovascular diseases estimated in 2012 by IOZ, defined as the cost of drugs incurred by NHF and patient, were PLN 8.85 billions. A significant amount of treatment cost are costs of treatment of acute coronary syndromes.
The authors of the publication Maciejewski 2009 estimated the cost of hospitalization of patients with ACS in Poland for PLN 5.4 billions in 2008, assuming 140 thousand hospitalizations per year. The results seem to be overestimated, taking into consideration the value stated for the whole group of cardiovascular diseases. According to NHF data in 2016 cost of hospitalization due to MI and unstable angina pectoris (ICD codes-10: I20, I21, I22) was PLN 1.3 billions of which 62% were costs of hospitalizations due to MI.[42]
Costs of ACS treatment were also estimated based on the data from PL-ACS registry.[43] This estimation uses data on the course of treatment of patients hospitalized at the turn of 2003/2004 and their 12-month observation period. Costs of hospitalization, drugs and outpatient care were considered. Mean annual cost of treatment in 2008 was PLN 12,526 for STEMI, PLN 11,428 for NSTEMI and PLN 9164 for the unstable coronary disease. Cost of hospitalization for conservative treatment (PLN 2387) was lower than cost of hospitalization for treatment with invasive method (PLN 8531).[37] Taking into consideration data of NHF from 2013 and costs from Gąsior 2008 study adjusted for inflation rate, annual cost of treatment in Poland of patients with first ACS in 2013 may be assessed for PLN 1.2 billion. In years of collecting data for estimation of costs, the percentage of treatment with invasive method was lower than currently. It should be expected that currently mean cost of ACS treatment is higher than reported in Gąsior 2008 study.[42]
Cost of MI hospitalization constituted 62% of ACS hospitalization costs.[42] According to data from NIZP-PZH report, NHF cost associated with the treatment of patients with MI in 2009 was PLN 1.3 billion in the first year and PLN 1.54 billion within 3 years.[37] Cost of the first hospitalization of patients with MI in 2009 was approximately PLN 875 million what gives a mean of PLN 11.4 thousand per patient per episode. After the first hospitalization until the end of first-year expenditure associated with further diagnostics and treatment after experienced MI in these patients were PLN 417 million. The additional cost of reimbursement of drugs associated with cardiovascular diseases was approximately PLN 33.5 million. In two consecutive years, NHF expenditure for treatment of patients after experienced MI was approximately PLN 202 million for health services and PLN 27 million for drugs.
Indirect costs of MI estimated by NIZP-PZH included loses due to productivity decrease (due to death or inability to work) and loss due to social transfers (loses are due to necessary distribution and transfers of parts of welfare from some people to other). Total value of loses qualified for indirect costs incurred due to MI which occurred in 2009 were estimated for amounts from PLN 3.1 to PLN 6 billion.[37]
Contribution of patients with FH in epidemiology of ACS
Diagnostics of patients with ACS in a medical history for FH was conducted in 5 European studies [27],[30],[31],[32],[44] and 3 studies from outside Europe.[45],[46],[47] FH diagnosis in the majority of studies was defined based on DLCN criteria for diagnosis probable/ definite FH (DLCN≥6). Studies population differ in terms of inclusion criteria although all the studies admitted patients with ACS in an interview. The reported percentage of patients with FH in the studies differ and amounts from 0.4% to 20.3% (Table 2). Low percentage in Rerup 2016 study is most likely due to lack of data for two criteria in DLCN scale. High FH percentage in Rallidis 2016 study results from the inclusion of patients with premature arteriosclerosis, who are more often diagnosed with familial hypercholesterolemia, into the study. A high percentage of patients with FH observed in Pang 2015 study[47] as well as in Rallidis 2016 may be due to excluding older patients. The population of EUROASPIRE IV study were patients under 80 years of age with heart episode in a medical history. In the study group, familial hypercholesterolemia was diagnosed in 8.3% patients. Standardised percentage of patients with FH in Polish population participating in EUROASPIRE IV trial was 11.4%.[27]
Table 2. Percentage of patients with FH in a group of patients with ACS in the interview
|
Trial |
Percentage of patients with FH |
N |
Country |
Studied population |
FH diagnosis criterion |
|
Europe |
|||||
|
De Backer 2015 (EUROASPIRE IV)[27] |
11.4%* |
357 |
Poland |
Patients hospitalized due to heart episode (ACS or revascularization) |
Probable/ definite FH in line with DLCN
|
|
8.3% |
7044 |
Europe (24 countries) |
|||
|
Mortensen 2016[44] |
2.0% |
1381 |
Denmark
|
Patients with first MI |
|
|
Rerup 2016[31] |
0.4%† |
13 174 |
Patients directed for angiography after myocardial infarction |
||
|
Nanchen 2016[30] |
1.6% |
4534 |
Switzerland |
Patients after ACS |
|
|
Rallidis 2016[32] |
20.3% |
320 |
Greece |
Patients after myocardial infarction at the age of <35 |
|
|
Other countries |
|||||
|
Li 2016[45] |
3.9% |
1843 |
China |
Patients directed for angiography after first myocardial infarction |
Probable/ definite FH in line with DLCN
|
|
Ohmura 2017[46] |
5.7% |
296 |
Japan |
Patients after ACS |
According to Japanese Cardiac Society occurrence of 2 out of 3 factors: LDL-C without treatment > 4.65 mmol/L (180 mg/dL), tendinous xanthoma, FH or premature ischemic heart disease in an interview |
|
Pang 2015[47] |
14.3% |
175 |
Australia |
ACS in the interview, ischemic disease or revascularization before the age of 60 |
Probable/ definite FH in line with DLCN
|
* Standardised to the age of general population of the study De Backer 2015;[27] † diagnosis does not consider 2 criteria of DLCN scale, i.e. physical study and family interview due to lack of such data in the register
Estimations of ACS costs in patients with FH in Poland
Costs of treatment of ACS in patients with FH were estimated based on the contribution of patients with FH among patients with ACS (Table 2). Mean percentage reported in EUROASPIRE IV trial was used due to the size of the trial, consideration of the patients from a broad range of age and presence of Polish population in the study. Additionally, the cost was estimated based on percentage reported only for Polish group (supplemental estimation due to small size subpopulation from Poland). The total cost of hospitalization due to ACS among patients with FH was in 2016 PLN 108-148 million, including the cost of hospitalization due to MI of PLN 67-91 million (Table 3). Direct medical cost during first 12 months after myocardial infarction in patients with FH may be estimated for approximately PLN 109-149 million and during first 36 months – for PLN 128-175 million. The indirect cost of MI in a population with FH may be estimated from PLN 257-353 million (lower range) do PLN 498-684 million (upper range).
Table 3. Estimated costs of ACS and MI in Poland in patients with FH
|
Event |
Cost description |
Estimation year |
Costs in Poland, in general [PLN] |
Costs for subpopulation with FH in Poland (based on mean distribution estimation of all patients with FH from EUROASPIRE IV trial) [PLN] |
Costs for subpopulation with FH in Poland (based on mean distribution estimation based on Polish data from EUROASPIRE IV trial) [PLN] |
|
Variant I: Own estimation based on DRG NHF data from 2016[42] on costs and distribution estimation of patients with FH in ACS and MI in EUROASPIRE IV trial |
|||||
|
ACS |
Total cost of hospitalization
|
2016
|
1,302,052,916 |
108,070,392 |
148,434,032 |
|
MI |
801,568,904 |
66,530,219 |
91,378,855 |
||
|
Variant II: Own estimation based on unit costs from PL-ACS registry (Gąsior 2008 study)[35], (publication of adjusted to 2013 for inflation rate and number of events in 2013 based on data from health needs maps (Więckowska 2015)[36] |
|||||
|
ACS |
Cost of treatment of patients with first ACS.MI episode
|
2013
|
1,249,452,512 |
103,704,558 |
142,437,586 |
|
MI |
872,833,279 |
72,445,162 |
99,502,994 |
||
|
Variant III: Estimations in NIZP-PZH report (based on data from AMI-PL base)[37] |
|||||
|
MI (during first 12 months) |
Costs of the first hospitalization |
2009
|
856,697,023 |
71,105,853 |
97,663,461 |
|
Cost of health services (excluding the first hospitalization) |
417,669,281 |
34,666,550 |
47,614,298 |
||
|
Cost of drugs |
33,501,507 |
2,780,625 |
3,819,172 |
||
|
Total medical direct costs |
1,307,897,811 |
108,555,518 |
149,100,350 |
||
|
MI (during first 36 months) |
Cost of health services (excluding the first hospitalization) |
619,747,388 |
51,439,033 |
70,651,202 |
|
|
Costs of drugs |
60,758,837 |
5,042,983 |
6,926,507 |
||
|
Total medical direct costs |
1,537,203,248 |
127,587,870 |
175,241,170 |
||
|
MI |
Indirect costs lower range upper range |
2009-2030
|
3,100,000,000 6,000,000,000 |
257,300,000 498,000,000 |
353,400,000 684,000,000 |
Ischemic strokes (IS)
Increased risk of IS in patients with FH vs general population
Clinical trials conducted in the last century, before introduction of statins, indicate a significant increase of risk of IS occurrence in patients with FH.[48],[49]What is more, 20-fold risk increase was demonstrated in FH population vs the general population.[50] Experts indicate on the association of high LDL cholesterol level and low HDL cholesterol level in blood in patients with familial hypercholesterolemia with increased occurrence of IS in these patients.[49] In SHEP[51] study each 15 mg/dL HDL cholesterol increase resulted in decreased risk of IS in patients above 60 years of age with isolated systolic blood pressure (RR 0.82; 95% CI: 0.69; 0.95). Meta-analysis of results of 27 clinical trials concentrated on the impact of decreased LDL cholesterol level in blood in patients with hypercholesterolemia on cardiovascular diseases demonstrated that every 1.0 mmol/L LDL-C cholesterol level decrease results in 15% decrease of IS risk (RR 0.85; 95% CI: 0.80; 0.89).[52] The latest studies on evolocumab (PCSK9 inhibitor) also demonstrated decreased risk of IS in patients with hypercholesterolemia after decreasing LDL-C cholesterol level [53] due to the proven association of increased LDL cholesterol level in blood with an increased risk of IS it is believed that patients with familial hypercholesterolemia have the increased risk of stroke. Probably this relationship is not as strong as in case of MI what is indicated by INTERHEART and INTERSTROKE studies. Nevertheless, complete elimination of lipid disorders could result in a ¼ decrease of a number of strokes (and ½ in case of MI).[54],[55]
Epidemiology of IS in Poland
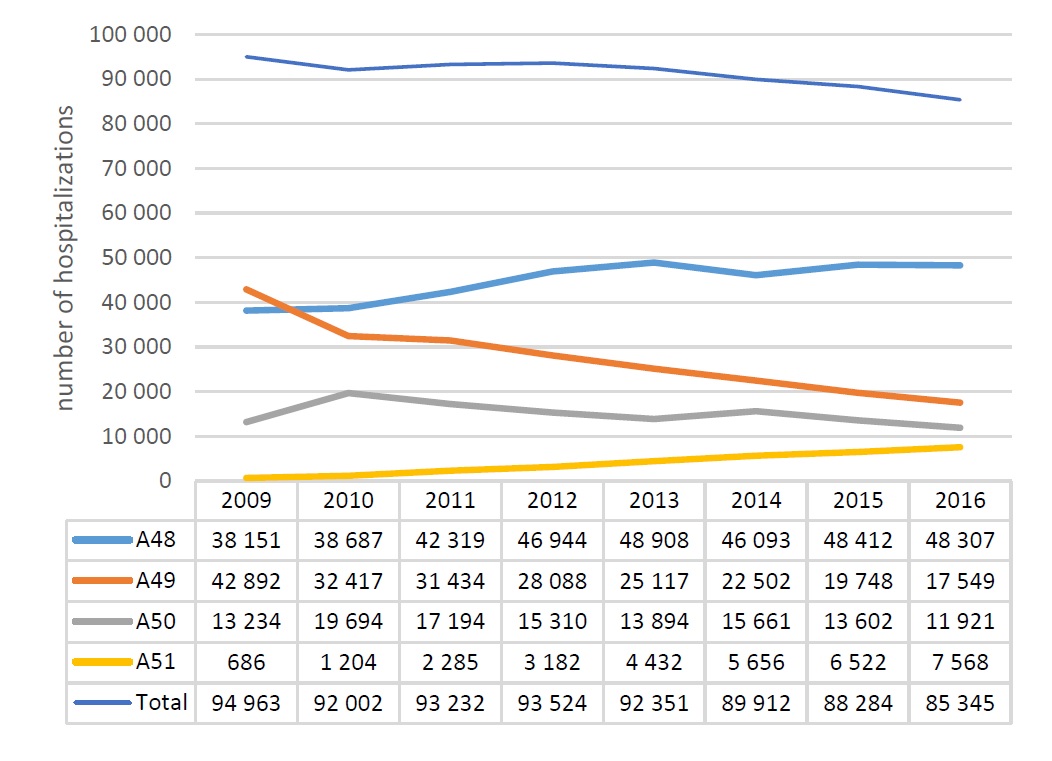
In Poland each year 80 thousand people are diagnosed with a stroke which in 24 thousand cases causes death within a year and in 32 thousand – permanent disability symptoms.[56] In 2010 IS incidence in Poland equaled 173.2 per 100 thousand inhabitants what resulted in the death rate of 51.1 per 100 thousand inhabitants and loss of 682.8 disability adjusted life-years per 100 thousand inhabitants (DALY, 1 DALY means loss of 1 year in health whose cause may be premature mortality of disablement).[57] A number of hospitalizations associated with strokes in Poland seem to decrease and in 2016 equaled 85.3 thousand (Figure 1). However, they still constitute the second, after ischemic heart disease, cause of death, both worldwide and in Poland.[58] Due to the ageing of society inhibition of decline trend in stroke incidence is anticipated. It is estimated that, if incidence did not change, the number of strokes in Poland in 2025 would increase to 96 thousand.[59]
Figure 1. Number of hospitalizations due to stroke for homogeneous patient groups A48 – A51 in years 2009-2016 in Poland [42],[57]
* Strokes are accounted for by the National Health Fund within homogeneous patient groups – A48, A49, A50 and A51.
Costs of IS in Poland
Strokes are accounted for by NHF within Diagnosis Related Groups (A48 Complex treatment of strokes > 7 days in a stroke unit, A49 - Stroke - treatment > 3 days, A50 - Stroke – treatment, A51 - Stroke - thrombolytic treatment > 7 days in stroke unit). In 2016 more than a half of cases (56.6%) was accounted for within group A48. Distribution of IS in a general number of hospitalizations within this group equaled 95%. Costs incurred by NHF for hospitalizations within four DRG groups in 2016 equaled more than PLN 656.5 million, of which 95% (PLN 621.4 million) were costs of IS treatment.[42]
Patients should begin rehabilitation under control of physiotherapist during 24 hours since stroke onset. This improves patient’s quality of life and decreases the risk of death through eliminating complications.[60] Experts estimate that rehabilitation after stroke in Poland constitutes approximately 60% of neurological rehabilitation. In 2015 PLN 318 million was spent on such services.[56]
No data exists on indirect costs incurred due to strokes in Poland. Expenditure associated with disability to work benefits as a result of cerebral vessels diseases (ICD-10: I60–I69) in 2014 reached PLN 1 billion (including: PLN 300.1 million – disability to work and independent existence; PLN 304 million – complete inability to work; PLN 430.2 million – partial inability to work). Expenses incurred as an effect of disease absence due to stroke in 2014 are estimated at PLN 61.4 million.[56]
No studies in which distribution of patients with FH in IS epidemiology was estimated precisely were found therefore total costs of treatment of strokes were presented without distinguished costs associated with IS in the course of FH.
Peripheral artery disease (PAD)
Increased risk of PAD in patients with FH vs general population
In patients with heterozygous familial hypercholesterolemia the increased risk of peripheral artery disease in comparison with the general population is observed what is clearly confirmed by among others cross-sectional study in Brazilian population (Pereira 2015) and Spain multicentre cohort study (Pérez de Isla 2016), both conducted in recent years.[23],[25]
Population in Pereira 2015 study consisted of 726 patients, including 202 with diagnosed FH. In Pereira 2015 study PAD was identified in approximately 1 per 5 patients with FH what means an almost 6-fold increase of risk of PAD occurrence in comparison with people with a correct level of lipids in blood (Table 4).[25] Pérez de Isla 2016 study was conducted in a population with genetically confirmed heterozygous FH. Patients were examined for atherosclerotic cardiovascular disease (ASCVD). The study demonstrated a 7-fold increase of PAD occurrence in patients with FH (1.4%) vs control group (0.2%) (Table 4).
Differences between the estimated frequency of PAD in discussed studies may be due to various sizes of studied groups, differences in clinical and laboratory characteristics of population and age differences among studies populations. In both discussed studies age was a significant factor associated with frequency of PAD occurrence.[23],[25] In Pérez de Isla 2016 study population median age was 44 and 40 years respectively in a group of patients with FH and control group. Pereira 2015 study population was characterised by mean age of 50.8 and 43.6 years respectively. According to the authors of both of these studies, collected data enable stating statistically significant increase in the frequency of occurrence of PAD in patients with FH vs the general population.
PAD epidemiology in Poland
Report on the peripheral artery and venous diseases (Andziak 2015) indicate an increase of PAD cases in Poland along with the increase of population age.[61] The frequency of lower limbs atherosclerosis in Poland in the total population is 3-10% increasing to 15-20% in people aged 70 and older. Atherosclerosis was the cause of 31.4 thousand deaths in Poland in 2010 what is 18% of deaths due to circulatory causes. The real death rate due to atherosclerosis was 87/100 000 inhabitants in 2009 and 82.2/100 000 inhabitants in 2010.[61]
World epidemiological data on PAD indicate that in high-income countries, including Poland, PAD frequency increase with age: from 5% for people aged 45-49 to 18% for people aged 85-89 and does not differ significantly among men and women.[62] In years 2000-2010 13% increase in a number of PAD cases in high-income countries was observed. This data is in accordance with data presented above for the Polish population.
In Dorobisz 2005 study atherosclerosis obliterans of lower limbs incidence in a population of Opole Voivodeship in years 1992 - 2002 was analysed (Table 5).[63] During the analysed years clearly visible increasing trend in atherosclerosis obliterans of lower limbs incidence is observed. It may be assumed that incidence trend sustained in the following years however due to long period of time from the publication of the discussed study and lack of newer data availability, number of new cases in a scale of country was stated based on recent available data (incidence 47.0/100 inhabitants) and current population of Poland (38,433 thousand of people [64]). A number of new cases stated in such a way in Poland equals approximately 18 thousand per year.
Table 4. Occurrence of PAD in patients with FH and in patients with correct level of lipids in blood
|
Study |
Number of patients with FH and PAD / number of patients with FH (% of patients with FH) |
Number of patients with PAD in control group / number of patients in control group (% of control group) |
p-value |
|
Pereira 2015 [25] |
35/202 (17.3) |
12/524 (2.3) |
<0.001 |
|
Pérez de Isla 2016 [23] |
39/2752 (1.4) |
2/993 (0.2) |
<0.001 |
Table 5. Atherosclerosis obliterans of lower limbs incidence in Opole Voivodeship (mean number of newly registered cases per 100 thousand inhabitants) [63]
|
Year |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
|
Incidence (/100 thousand inhabitants) |
24.5 |
35.5 |
41.2 |
42.7 |
41.9 |
43.7 |
42.3 |
41.6 |
41.8 |
41.8 |
47.0 |
PAD costs in Poland
No published data on PAD costs in Poland exists. An attempt to estimate the cost of hospitalization due to PAD was made based on homogeneous patient groups of the National Health Fund system statistics from 2016 (Table 6).[65] Cost of hospitalization due to PAD was calculated based on data available for the following codes: ICD-10: I70.1 – arteriosclerosis of kidney artery, I70.2 – arteriosclerosis of lower limbs, I70.9 – generalized and indefinite arteriosclerosis and I73.9 – peripheral artery disease, undefined. Estimated number of hospitalizations associated with PAD was 74,246, and their total cost was approximately PLN 373,269,152.
Apart from direct costs of hospitalization also social costs of non-treatment or incorrect treatment of arteriosclerosis are significant. Patients with amputations in numerous cases became unable to work, often require constant care by the third person, and only 10 to 15% efficiently receives a prosthesis.[61]
No studies precisely estimating the contribution of patients with FH in PAD epidemiology were found therefore total costs of PAD treatment are presented without distinguished costs associated with PAD in the course of FH.
Table 6. Presentation of a number and costs of hospitalizations for peripheral artery disease based on DRG statistics (the Year 2016 - Catalogue 1a) [65]
|
ICD-10 code |
Mean values of hospitalizations according to ICD-10 code [PLN] |
Number of hospitalizations |
Total costs of hospitalizations [PLN] |
|
I70.1 |
3667.65 |
69 |
244,681.50 |
|
I70.2 |
7889.81 |
44,036 |
293,395,351.93 |
|
I70.9 |
7251.56 |
30,055 |
78,099,033.05 |
|
I73.9 |
10,717.89 |
86 |
1,530,085.46 |
|
MEAN |
7381.73 |
- |
- |
|
TOTAL |
- |
74,246 |
373,269,151.94 |
Conclusions
The most important conclusions from the article were summarized in Table 7. Heterozygous familial hypercholesterolemia concerns more than 150,000 of Poles. In the majority of cases, the disease remains undiagnosed. Patients with FH are at increased risk of premature coronary disease and complications such as ACS, MI, ischemic stroke or peripheral artery disease. Early diagnosis of familial hypercholesterolemia and implementation of efficient hypolipemic treatment has a potential of significant decrease in costs incurred in the healthcare system.
Table 7. Summary of the most important conclusions from the article
|
FH and its complications |
|
|
FH epidemiology in Poland |
|
|
FH complications cost in Poland |
|
Conflict of interest: This study was supported by Sanofi – Aventis Sp. z o.o., Warsaw, Poland.
[1] Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., Hoes A.W., Jennings C.S., Landmesser U., Pedersen T.R., Reiner Z., Riccardi G., Taskinen M.R., Tokgozoglu L., Verschuren W.M., Vlachopoulos C., Wood D.A., Zamorano J.L.: Guidelines for the management of dyslipidaemias. Eur. Heart. J. 2016; 37(39): 2999-3058.
[2] Idzior-Walus B., Sanak M., Starzyk J., Czarnecka D., Walus-Miarka M.: Autosomalna dominująca hipercholestreolemia – niedoceniony problem diagnostyczny i kliniczny. Kardiol. Pol. 2009; 67: 1015-1022.
[3] Rynkiewicz A., Cybulska B., Banach M., Filipiak K.J., Guzik T., Idzior-Waluś B., Imiela J., Jankowski P., Kłosiewicz Latoszek L., Limon J., Myśliwiec M., Opolski G., Steciwko A., Stępińska J., Zdrojewski T.: Postępowanie w heterozygotycznej hipercholesterolemii rodzinnej. Stanowisko Forum Ekspertów Lipidowych. Kardiol. Pol. 2013; 71(1): 107–111.
[4] Henderson R., O’Kane M., McGilligan V., Watterson S.: The genetics and screening of familial hypercholesterolaemia. Journal of Biomedical Science. 2016: 23-39.
[5] Cuchel M., Bruckert E., Ginsberg H.N., Raal F.J., Santos R.D., Hegele R.A., Kuivenhoven J.A., Nordestgaard B.G., Descamps O.S., Steinhagen-Thiessen E., Tybjærg-Hansen A., Watts G.F., Averna M., Boileau C., Borén J., Catapano A.L., Defesche J.C., Hovingh G.K., Humphries S.E., Kovanen P.T., Masana L., Pajukanta P., Parhofer K.G., Ray K.K., Stalenhoef A.F., Stroes E., Taskinen M.R., Wiegman A., Wiklund O., Chapman M.J.: European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014; 35(32): 2146-57
[6] Nordestgaard B.G., Chapman MJ, Humphries S.E.: European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013; 34(45): 3478-90a.
[7] Akioyamen L.E., Genest J., Shan S.D., Reel R.L., Albaum J.M., Chu A., Tu J.V.: Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open. 2017; 7(9): e016461.
[8] Homsma S.J., Huijgen R., Middeldorp S., Sijbrands E.J., Kastelein J.J.: Molecular screening for familial hypercholesterolaemia: consequences for life and disability insurance. Eur J Hum Genet. 2008;16(1): 14-7.
[9] Reiner Z., Catapano A.L., De Becker G., Taskinen M.R., Wiklund O., Agewall S., Alegria E., Chapman M.J., Durrington P., Erdine S., Holcox J., Hobbs R., Kjekshus J., Filardi P.P., Riccardi G., Storey R.F., Wood D.: Wytyczne ESC/EAS dotyczące postępowania w dyslipidemiach. Kardiol. Pol. 2011; 69, supl. IV: 143–200.
[10] Rywik S., Pająk A.: Monitoring of cardiovascular incidence, fatality and mortality trends and their determinants – longitudinal study Pol MONICA. Part III: principles of standardization and quality control. Przegl. Lek. 1985; 42: 280-284.
[11] Rywik S., Sznajd J., Kulesza, W.: Monitoring of cardiovascular incidence, fatality and mortality trends and their determinants – longitudinal study Pol MONICA. Part II: material and methods. Przegl. Lek. 1985; 42: 256-260.
[12] Rywik S., Kupść W., Piotrowski W., Broda G., Piwoński J.: Wieloośrodkowe ogólnopolskie badanie stanu zdrowia ludności – projekt WOBASZ. Założenia metodyczne oraz logistyka. Kardiol. Pol. 2005; 63: S1–S8.
[13] Pająk A., Wiercińska E., Polakowska M., Kozakiewicz K., Kaczmarczyk-Chałas K., Tykarski A., Gaździk D., Zdrojewski T.: Rozpowszechnienie dyslipidemii u mężczyzn i kobiet w wieku 20–74 lat w Polsce. Wyniki programu WOBASZ. Kardiol. Pol. 2005; 63: 6 (supl. 4).
[14] Peasey A., Bobak M., Kubinova R.: Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: rationale and design of the HAPIEE study. BMC Public Health 2006; 6: 255-60.
[15] Zdrojewski T., Rutkowski M., Bandosz P.: Prevalence and control of cardiovascular risk factors in Poland. Assumptions and objectives of the NATPOL 2011 Survey. Kardiol. Pol. 2013; 71, 4: 381-392.
[16] Pajak A., Szafraniec K., Polak M., Drygas W., Piotrowski W., Zdrojewski T., Jankowski P.: Prevalence of familial hypercholesterolemia: a meta-analysis of six large, observational, population-based studies in Poland. Arch Med Sci. 2016; 12(4): 687-96.
[17] Stępińska J., Solnica B., Kulpa J., Jankowski P., Kalarus Z., Opolski G., Sitkiewicz D.: Konieczność ujednolicenia wartości docelowych wyników badań lipidowych w medycznych laboratoriach diagnostycznych w Polsce. Journal of Laboratory Diagnostics 2012; 48, 4: 473-474.
[18] Cieśliński A., Pająk A., Podolec P., Rynkiewicz A.: Ogólnopolski Program Prewencji Choroby Wieńcowej POLSCREEN. Wydawnictwo Termedia, Poznań 2016.
[19] Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., Graham I., Hall M.S., Hobbs F.D., Løchen M.L., Löllgen H., Marques-Vidal P., Perk J., Prescott E., Redon J., Richter D.J., Sattar N., Smulders Y., Tiberi M., Bart van der Worp H., van Dis I., Verschuren W.M.: 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016; 252: 207-74.
[20] Austin M.A., Hutter C.M., Zimmern R.L., Humphries S.E.: Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol. 2004; 160(5): 421-9.
[21] Hopkins P.N., Toth P.P., Ballantyne C.M., Rader D.J.: National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011; 5(3 Suppl): S9-17.
[22] Gencer B., Nanchen D.: Identifying familial hypercholesterolemia in acute coronary syndrome. Curr Opin Lipidol. 2016; 27(4): 375-81.
[23] Pérez de Isla L., Alonso R., Mata N., Saltijeral A., Muñiz O., Rubio-Marin P., Diaz-Diaz J.L., Fuentes F., de Andrés R., Zambón D., Galiana J., Piedecausa M., Aguado R., Mosquera D., Vidal J.I., Ruiz E., Manjón L., Mauri M., Padró T., Miramontes J.P., Mata P.; SAFEHEART Investigators. Coronary Heart Disease, Peripheral Arterial Disease, and Stroke in Familial Hypercholesterolaemia: Insights From the SAFEHEART Registry (Spanish Familial Hypercholesterolaemia Cohort Study). Arterioscler Thromb Vasc Biol. 2016; 36(9): 2004-10.
[24] Kjærgaard K.A., Christiansen M.K., Schmidt M., Olsen M.S., Jensen H.K.: Long-Term Cardiovascular Risk in Heterozygous Familial Hypercholesterolemia Relatives Identified by Cascade Screening. J Am Heart Assoc. 2017; 6(6): e005435.
[25] Pereira C., Miname M.H., Makdisse M.R., Watanabe C., Pesaro A.E., Jannes C.E., Kalil Filho R., Pereira A.C., Santos R.D.: Peripheral arterial disease in heterozygous familial hypercholesterolemia. Atherosclerosis. 2015; 242(1): 174-8.
[26] Wilkins E., Wilson L., Wickramasinghe K., Bhatnagar P., Leal J., Luengo-Fernandez R., Burns R., Rayner M., Townsend N.: European Cardiovascular Disease Statistics 2017. European Heart Network, Brussels 2017.
[27] De Backer G., Besseling J., Chapman J.: Prevalence and management of familial hypercholesterolaemia in coronary patients: An analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis. 2015; 241: 169–175.
[28] Krogh H.W., Mundal L., Holven K.B.: Patients with familial hypercholesterolaemia are characterized by presence of cardiovascular disease at the time of death. Eur Heart J 2016; 37: 1398–1405.
[29] Khera A.V., Won H.H., Peloso G.M.: Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016; 67: 2578–2589.
[30] Nanchen D., Gencer B., Muller O.: Prognosis of patients with familial hypercholesterolemia after acute coronary syndromes. Circulation. 2016; 134: 698–709.
[31] Rerup S.A., Bang L.E., Mogensen U.M.: The prevalence and prognostic importance of possible familial hypercholesterolemia in patients with myocardial infarction. Am Heart J. 2016; 181: 35–42.
[32] Rallidis L.S., Triantafyllis A.S., Tsirebolos G.: Prevalence of heterozygous familial hypercholesterolaemia and its impact on long-term prognosis in patients with very early ST-segment elevation myocardial infarction in the era of statins. Atherosclerosis. 2016; 249: 17–21.
[33] Mundal L., Sarancic M., Ose L., Iversen P.O., Borgan J.K., Veierod M.B., Leren T.P., Retterstol K.: Mortality among patients with familial hypercholesterolemia: a registry-based study in Norway, 1992–2010. J Am Heart Assoc. 2013; 3: e001236.
[34] Ministerstwo Zdrowia. Mapa potrzeb zdrowotnych w zakresie kardiologii dla Polski. Warszawa 2015.
[35] Gąsior M., Pres D., Wojakowski W., Buszman P., Kalarus Z., Hawranek M., Gierlotka M., Lekston A., Mizia-Stec K., Zembala M., Poloński L., Tendera M.: Causes of hospitalization and prognosis in patients with cardiovascular diseases. Secular trends in the years 2006-2014 according to the SILesian CARDiovascular (SILCARD) database. Pol Arch Med Wewn. 2016; 126(10): 754-762.
[36] Więckowska B.: Proces leczenia w Polsce – analizy i modele. Tom II: Kardiologia. Ministerstwo Zdrowia, Warszawa 2015.
[37] Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny. Raport: Występowanie, leczenie i prewencja wtórna zawałów serca w Polsce. Ocena na podstawie Narodowej Bazy Danych Zawałów Serca AMI-PL 2009-2012. Warszawa 2014.
[38] Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny. Sytuacja zdrowotna ludności Polski i jej uwarunkowania. Warszawa 2012.
[39] KMPG w Polsce. Analiza zmian społeczno-demograficznych oraz wpływu złego odżywiania, niedostatecznej aktywności fizycznej, nałogów i innych czynników ryzyka na rozpowszechnienie oraz koszty cukrzycy i chorób sercowo-naczyniowych w Polsce. Stan obecny i prognoza do 2030 roku. Raport przygotowany we współpracy z Fundacją Nutricia.
[40] Nichols M., Townsend N., Luengo-Fernandez R.: European Cardiovascular Disease Statistics 2012. European Heart Network, Brussels, European Society of Cardiology, Sophia Antipolis 2012.
[41] Raport Instytutu Ochrony Zdrowia. Zdrowie priorytetem politycznym państwa - analiza i rekomendacje. Warszawa 2013.
[42] Opracowanie własne na podstawie danych NFZ katalog JGP za rok 2016
[43] Gąsior U.: Ocena kosztów leczenia ostrych zespołów wieńcowych (OZW). Farmaceutyczny Przegląd Naukowy. 2008; 4: 43-44.
[44] Mortensen M.B., Kulenovic I., Klausen I.C.: Familial hypercholesterolemia among unselected contemporary patients presenting with first myocardial infarction: Prevalence, risk factor burden, and impact on age at presentation. J Clin Lipidol. 2016; 10: 1145–1152.
[45] Li S., Zhang Y., Zhu C.G.: Identification of familial hypercholesterolemia in patients with myocardial infarction: a Chinese cohort study. J Clin Lipidol. 2016; 10: 1344–1352.
[46] Ohmura H., Fukushima Y., Mizuno A.: Estimated prevalence of heterozygous familial hypercholesterolemia in patients with acute coronary syndrome. Int Heart J. 2017; 58: 88–94.
[47] Pang J., Poulter E.B., Bell D.A.: Frequency of familial hypercholesterolemia in patients with early-onset coronary artery disease admitted to a coronary care unit. Journal of clinical lipidology. 2015; 9: 703–708.
[48] Bansal B.C., Sood A.K., Bansal C.B.: Familial hyperlipidemia in stroke in the young. Stroke. 1986;17: 1142-1145.
[49] Vuorio A.F., Kovanen P.T.: Do statins reduce the incidence of stroke in familial hypercholesterolemia? Expert Rev. Cardiovasc. Ther. 2011; 9(3): 349–353.
[50] Kaste M. Koivisto P.: Risk of brain infarction in familial hypercholesterolemia. Stroke. 1988; 19: 1097-1100.
[51] Ansell J.: Cholesterol, stroke risk and rtroke prevention. Curr Atheroscler Rep. 2000; 2: 92.
[52] Cholesterol Treatment Trialists (CTT) Collaboration: Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015; 385: 1397–1405.
[53] Sabatine M., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D.., Murphy SA., Kuder J.F., Wang H., Liu T., Wasserman S.M., Sever P.S., Pedersen T.R., FOURIER Steering Committee and Investigators.: Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017; 376: 1713-1722.
[54] Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators.: Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364: 937-52.
[55] O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S; INTERSTROKE investigators.: Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016; 388: 761-75.
[56] Strzelecki Z., Szymborski J.: Zachorowalność i umieralność na choroby układu krążenia a sytuacja demograficzna polski. Rządowa Rada Ludnościowa. 2015.
[57] Udary mózgu – rosnący problem w starzejącym się społeczeństwie. Raport Instytutu Ochrony Zdrowia. 2016.
[58] Dane WHO. 2015 [cytowane 29.09.2017]. Dostępne z: http://www.who.int/mediacentre/factsheets/fs310/en/
[59] Grabowska – Fudala B., Jaracz K. Górna K.: Zapadalność, śmiertelność i umieralność z powodu udarów mózgu – aktualne tendencje i prognozy na przyszłość. Przegl Epidemiol. 2010; 64: 439 – 442.
[60] Dane na temat rehabilitacji [cytowane 29.09.2017]. Dostępne z: http://www.udarowcy.com.pl/rehabilitacja
[61] Andziak P., Oszkinis G.: Choroby tętnic i żył obwodowych jako narastający problem zdrowotny starzejącego się społeczeństwa. Zachorowalność i umieralność na choroby układu krążenia a sytuacja demograficzna Polski. 2015; 157-170.
[62] Fowkes F.G., Aboyans V., Fowkes F.J., McDermott M.M., Sampson U.K., Criqui M.H.: Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017; 14(3): 156-170.
[63] Dorobisz A., Kucharski A., Sikorski A., Kowalik Z., Hobot J.: Zachorowalność na miażdżycę zarostową tętnic kończyn dolnych w populacji Opolszczyzny. Przegląd epidemiologiczny. 2005; 59: 933-944.
[64] Mały rocznik statystyczny Polski 2017. GUS 2017.
[65] Dane statystyczne. [cytowane 29.09.2017]. Dostępne z: https://prog.nfz.gov.pl/app-jgp/Start.aspx