Opioids, history still present. Policy issues in implementing the drug treatment of pain
-
Copyright
© 2012 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Wojciech Germaziak |
Home Medical Library, Warsaw, Poland |
In the history of medicine and pharmacy issues of treatment or prevention of pain disorders have been discussed since ancient times to the present. Opioids are a class of drugs controlled in the manufacturing, distribution, their use is specified by a certain health policy, medicine, pharmaceutical economics and rationalization of treatment within the competence of the ministries of health. The treatment of chronic pain management in both origin cancer and non-malignant forms provides solutions to ensure the relative balance in all aspects of the policy of balance, or balancing the risk factors associated with the use of opioids as controlled drugs in health care.
In the analysis of a variety of sources, including the evaluation of the Polish pharmaceutical market, particularly during the legislative process of the Reimbursement Act, it seems an important challenge for state drug policy makers to balance the controlled drugs market (including opioids).
The healing properties of opioids have been known to mankind for centuries. Poppy, due to the possibility of using both petals of flowers and seeds became a major source of these substances. Parts of the plant are used in the treatment of various diseases and ailments. In order to know the qualities and characteristics of opioid as a medicine with special properties better it is worth presenting the brief history of their use.
Poppy as the main component of herbal mixtures, called species pectorales already in ancient times, was identified as an excellent source of soothing coughs and ailments of the heart muscle [1]. The positive effects of it, especially in children drew the attention of the botanist Ignacy Czerwiakowski [2]. Chomel, on the other hand, discovered properties of poppy seeds, relieving pain caused by colic [3].
Positive impact of the substance on complaints was evident not only among circles of folk rymedicine. The use of the poppy by pharmacists confirmed in his work Józef Celiński who rysaid "In pharmacies [poppy leafs] are used for infusion, tincture and syrup” [4]. A record of the use of opioids in the form of herbs also appears in J. Trapp’s "Farmakognozyi" [5].
Poppy properties described by the researchers had their origin in opium - the substance harvested as milk poppy from immature green pericarp head of plants. Medicinal syrups and alcoholic tincture of the dried pouch were prepared. High quality of Polish opium was emphasized due to the morphine content of 12-13% [6] Apart from morphine more than 20 other alkaloids were detected in opium. Its derivatives, such as papaverine (spasmolytic) and codeine (antitussive) [7] have also been used in medicine.
Romuald Świerzbiński delivered a lot of important information on the effects of morphine in his publication of 1857. The author classified morphine as a drug to reduce pain and as a muscle relaxant. Effects of the substance on the circulatory system in the form of acceleration of pulse and breath, increase of temperature and pressure were found. In 1933, Jan Muszyński by analyzing a number of the formulations prepared on the basis of opium (including Extractum Opii, Opii crocata), identified narcotic substances as exclusive in medicine.
Opium was widely used over the next centuries. It has been often used as a antidiarrheal and diuretic. However the most well-known and appreciated were its pain-relieving and hypnotic properties. There were also documented cases of the use of opium in the treatment of alcoholism [8] and mental illnesses. The drug also demonstrated to be effective in dealing with people in hysteria or having trouble with insomnia. It was used during surgeries, childbirth, intestinal parasite control and treatment of malaria. It turned out to be known and appreciated as a detoxifying aid in cases of poisoning. Medical calendars of the following years provide accurate descriptions of a wide range of indications for the use of opium in the treatment of various disorders.
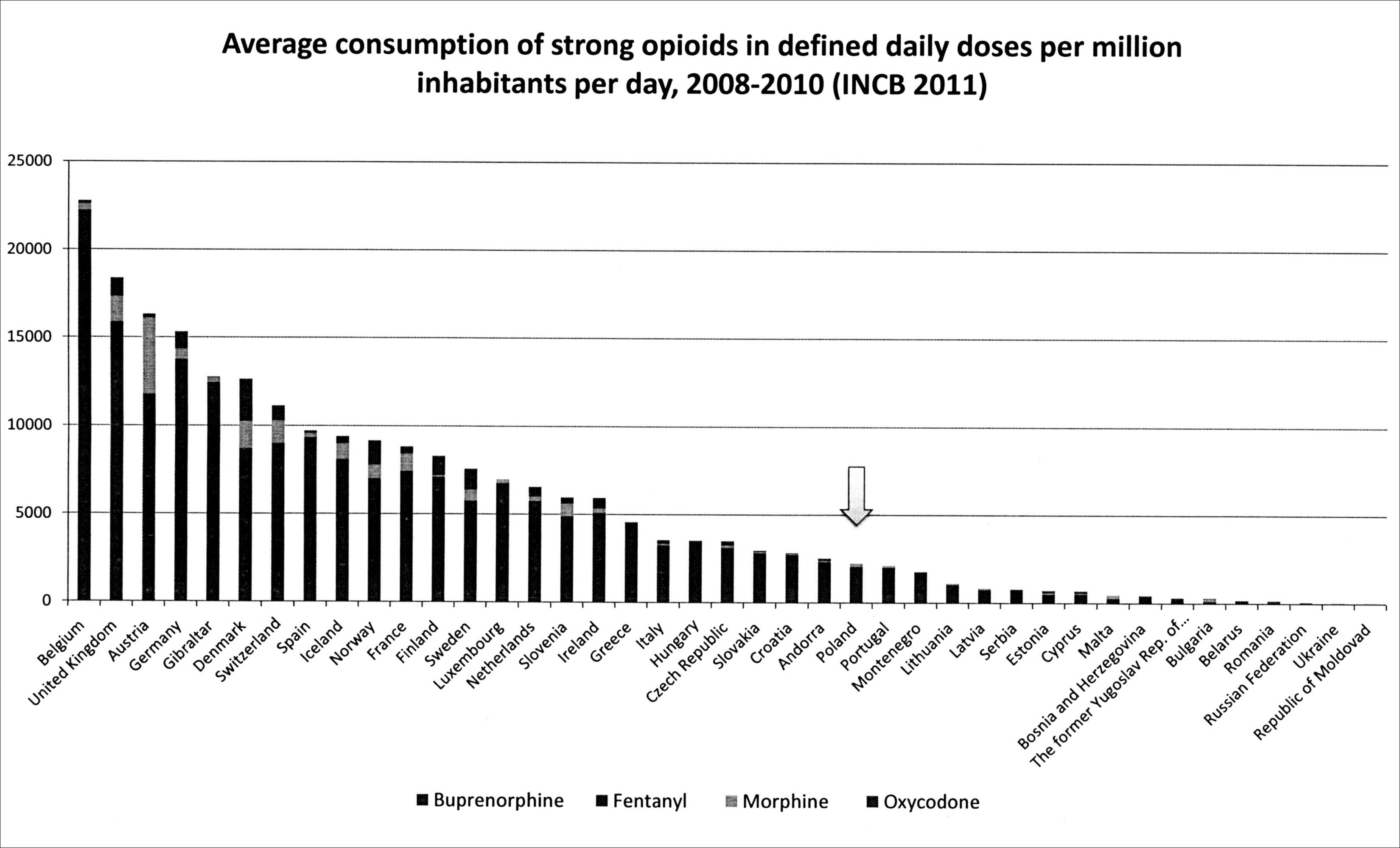
Despite the passage of time, progress in technology and medicine, opioids continue to play an important role in pharmacotherapy. Today’s focus is primarily on the use of analgesic properties of these substances. Opioid analgesics are mainly based on the treatment of cancer pain. Ever more frequently they are also helpful in relieving chronic (moderate and severe) non-malignant pain. The problem of use of "heavy opiates’ in daily doses in individual European countries is illustrated in the graph below on basis of data 2008-2010 INCB.
Cases of significant reduction of pain in patients with chronic non-cancer pain, continuing a long-term opioid therapy were confirmed by analysis of the Cochrane Collaboration [9].
From the beginning of the medical use of opioids both researchers and doctors have tried to determine and analyze the safety of opioid preparations., Leopold Lafontaine, the surgeon living in the nineteenth century stated that the administration of opium to children can be deadly toxic [10]. Similarly spoke Jan Biegański: „decoction of poppy puts to sleep (...), which in general badly affects health of children (...) and sometimes baby is put to sleep forever" [1]. It was feared that the inadequate doses of the drug can poison, which was also reflected in "Calendar for year 1921” containing descriptions of the treatment of excessive consumption of opioids [11].
Currently concerns relate mainly to the likelihood of opioid dependence. Physicians providing treatment direct their efforts to build an appropriate dosing regimen and careful monitoring of the progress, effectiveness and side effects of treatment implemented. Systematic review of the literature data show that less than 0.5% of patients with a negative history of substance abuse, committed an abuse of prescription pharmaceuticals while taking opioid analgesics [12]. This information is also confirmed by the study of Cochrane Collaboration which showed that only 0.27% of patients participating in the experiments with symptoms of dependence were observed [9].
The commonness of chronic non-malignant pain (every fifth European adult suffers from it, more frequently - the elderly) led scientists to begin the development of the research on pain and methods of management of it [13]. A group of experts in the field of pain was inter alia set up for this purpose - OPENMinds (Opioids and Pain European Network of Minds). This group with the publication of the "White Book” in 2005 and 2011 obliged European governments the to make policy changes into the treatment of pain and access to opioid drugs. The following are the key topics of the above publication.
Special attention was paid to the problem of pain in people with cancer. Study of European Pain in Cancer (EPIC) found that more than half of the above patients exhibit pain. Furthermore it was found that nearly 80% of patients experience chronic pain with the development of the disease. The research led to the following conclusions: about one third of patients were not treated at the time of the study, and 20% had never applied analgesics prescribed drugs. Only one in twenty patients was treated for pain with the help of a specialist [14].
International organizations such as the World Health Organization (WHO) and Human Rights Watch have qualified the access to pain treatment as one of the fundamental human and patient rights. Ensuring patient access to the highest quality of pain management systems came under the responsibility of national governments. Directives, adopted by the European Parliament, regulate international health care and patients’ rights to obtain the reimbursement in of the country of residence for health care received abroad [15].
As mentioned before, the pain has not been treated properly in most countries. The reasons for this situation are considered to be the low-quality and standard of prevention of pain and treatment conducted and marginalization of the problem of the chronic pain. According to the Declaration of Montreal as obstacles in gaining access to optimal health care system is inter alia lack of qualified medical personnel in the treatment of pain, a low level of scientific research on pain, limited access to opioids and other drugs used for pain [16]. According to the Achieving Balance in National Opioids Control 2011 guidelines, governments have a two-pronged obligation in the case f controlled drugs (such as opioids): firstly to ensure the availability of substances to be used for medical purposes and secondly they should protect patients against abuse of drugs and addiction to them [17].
The subject of controlled drugs carries a lot of problematic issues, such as the availability of these substances, affordability of them and above all, the control. The implementation of the policy related to this group of medications requires the support to governments and societies. According to WHO, this policy should be characterized by the effort to achieve a balance between the access, use of drugs and their abuse. The distribution and migration of opioids are subject to international laws and national drug control policies of individual countries. The government is burdened with the responsibility for balancing the risk of use of drug with an appropriate (optimal) access to treatment. Members of OPENMinds mark out a few areas of government policies aimed at improvement and development of the management of the treatment of chronic pain (of cancer and non-malignant origin).
These are the following issues:
- prioritizing the treatment of pain;
- raising the level of knowledge and skills of health staff through cooperation with national educational institutions;
- creation or maintenance of interdisciplinary outpatient clinics for the diagnosis and treatment of pain;
- ensuring the availability of analgesics (including opioids);
- improvement in pain prevention by promoting and funding research on the nature of pain;
- the establishment of appropriate facilities (resources) to achieve the proper level of safeguard for services.
To enable the achievement of the accepted goal of balanced levels of use, the World Health Organization has developed sui generis guide ("Ensuring the balance of national policies on the controlled substances: Recommendations for the availability and accessibility of the controlled medicines") in the form of recommendations for the optimization of the drug policy under control. Individual instructions can be grouped according to the aspect discussed.
The recommendations emphasize the importance of the rules, especially in the process of implementation, execution and development of the controlled drugs policy. The very first two recommendations concern legislation issues. According to the first of them, the drug control policy should be based on the necessary information about their use in the medical and research purposes. In accordance with article 2 of the Single Convention on Narcotic Drugs and article 5 of the Convention on Psychotropic Substances, governments should recognize the need for controlled drugs for the treatment of pain and for scientific purposes and take care of appropriate access to these substances. It seems important to improve the state laws to adopt international regulations. Recommendation nr 2 describes in detail the role of the heads of states and governments as to this issue. The obligation of governments to respect the law on the control over resources as well as other standards relating to human rights was emphasized. Human rights coupled with patients’ rights require the access to drugs (including opioids), under the right to health. Already in 2005 the World Health Assembly and the Economic and Social Council of the United Nations called the countries to follow international treaties in order to ensure access to and the use of opioid therapies. The guidelines contained in article 4 of the Uniform Convention are similar in tone: "The parties shall adopt such legislative and administrative measures which they consider necessary ... to limit the collection, production, distribution ..., ... the use and possession of drugs exclusively for medical and scientific purposes." Article 12 of the International Covenant on Economic, Social and Cultural Rights (ICESCR) raises such an issue as the right to access to the appropriate treatment of conditions ensuring the use of essential drugs, and also increases the importance of a balanced therapy with monitored drugs.
Recommendation no 7 indicates a major role of planning of the pharmaceutical policy in terms of availability and affordability of the controlled drugs. It justifies the need to include the issue of controlled drugs in the national control system and public health policy. Policy plans allow to lay down the appropriate target, the implementation of access to medicines, and monitoring of the fulfillment of obligations for states imposed by international conventions on the use of both narcotic drugs and patients’ rights. The importance of including the subject in policy plans affordability of drugs for scientific as well as medicinal purposes was emphasized. It is extremely important to include points associated with the availability of opioid analgesics as well as an integrated system of palliative care, especially in patients with cancer origin pain, in the program. The task of the government is to create the essential drugs list (based on the WHO List of Essential Medicines) - controlled drugs necessary to meet the immediate (medical) needs of the society. The policy carried out by governments should also include the strategy for optimal and rational use of the above mentioned drugs. An important issue is the distribution of reliable information on controlled drugs (social campaigns, leaflets).
International human rights law also imposes the obligation to respect the principle of non-discrimination on the availability of controlled drugs and the prevention of addictions of their use on the government. Drug policies lead by individual countries should take such actions which would ensure equal access to medicines for each group of people, regardless of economic, ethnic factors, etc. Each patient should have same rights to the use of drugs in therapies. The situation of patients using opioid analgesics is special, particularly with HIV positive patients, prisoners and opioid addicts. In many countries, these patients are still in some way discriminated – the access to controlled drugs in these cases is difficult or intentionally restricted. According to article 2 of the Universal Declaration of Human Rights, there is no reason to split patients into different groups of non-medical criteria, "every man has all the rights and freedom (...), regardless of race, color, sex, language, religion (...) or any other status, do not also make a difference depending on the political, jurisdictional or international status of the country or territory to which a person belongs (...) ".
Procedures related to the legislation should not regulate the issues relating to the use of controlled drugs too strictly. The task of government is to establish appropriate regulations, to optimize the use of drugs. Under the article 23 of the Convention on Psychotropic Substances it is permitted and acceptable to use more precise (tighter) control measures, if such measures are dictated by the need to protect public health. It seems important to verify the usefulness of legal norms in contribution to the protection of the public health. Situations in which the accepted legal rules constitute a barrier to accessibility (affordability) of drugs, but do not affect the reduction of their abuse, should be avoided. Keeping records of opioid-dependent patients, the detail of which can contribute to the difficulty of obtaining the drug for patients from more than one source may serve as an example of this type of the phenomenon... Other barriers such as affordability of medicines are due to the use in some countries, severe penalties for any errors or problems with prescriptions (drug release) of drugs under control. The effect of such legislation may be refraining from releasing such medicines by health care professionals.
These elements of the strategy (policy) of the controlled drugs constitute specific challenges for the governments of Europe, but it is worth making an effort to improve the current situation. There are also expectations that investments in these spaces of activities will bring measurable economic tangible benefits to individual countries due to the reduction of the indirect costs of the chronic pain, including productivity lost and informal care.